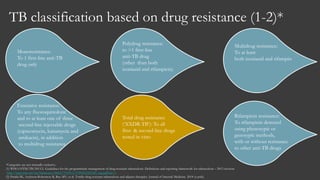
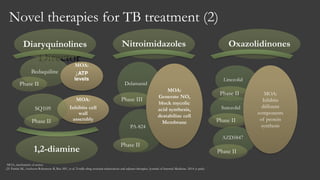
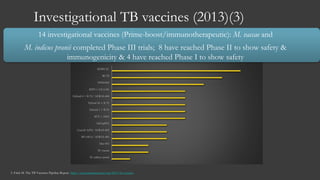
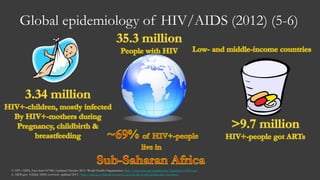
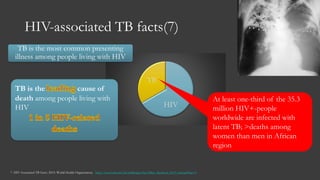
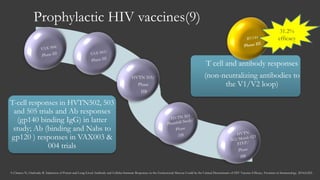
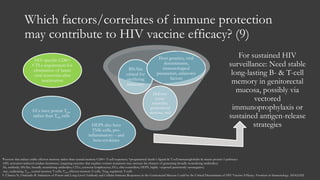
The document outlines key developments in HIV/TB research, including categorization of drug-resistant tuberculosis and novel therapies in development. It highlights significant findings on the global epidemiology of HIV/AIDS, particularly its association with tuberculosis, and the importance of effective treatment strategies. Additionally, it discusses the role of host genetic factors and the efficacy of investigational vaccines aimed at improving immune responses against HIV.