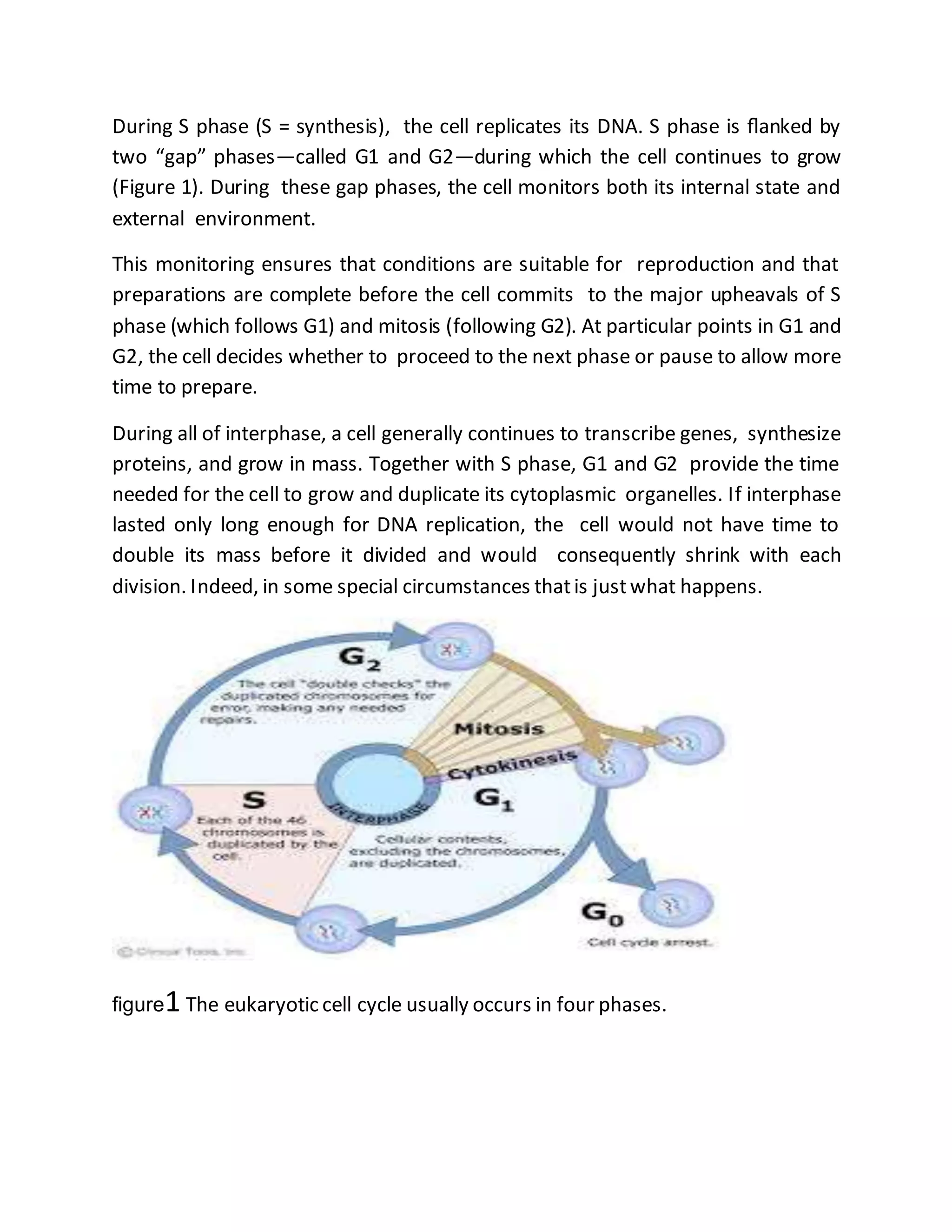
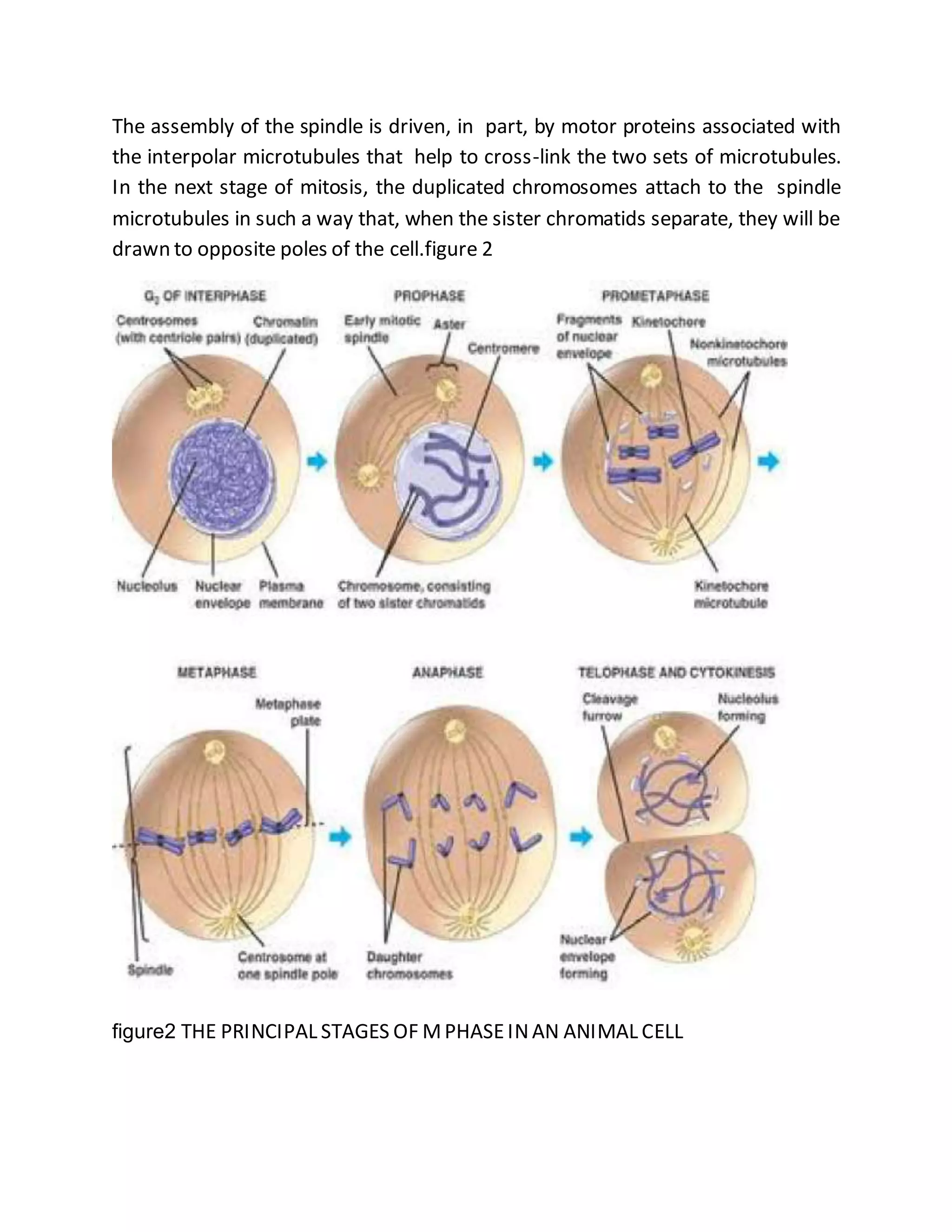
The cell cycle is a process by which cells duplicate their contents and divide, essential for reproduction in all living organisms, featuring phases such as interphase, mitosis, and cytokinesis. Regulatory proteins known as the cell-cycle control system ensure orderly progression through the cycle, checking for conditions favorable for DNA replication and cell division, which prevents issues like cancer. Mitosis involves the separation of duplicated chromosomes with the aid of the mitotic spindle, followed by cytokinesis, where the cytoplasm divides, completing the cell division.