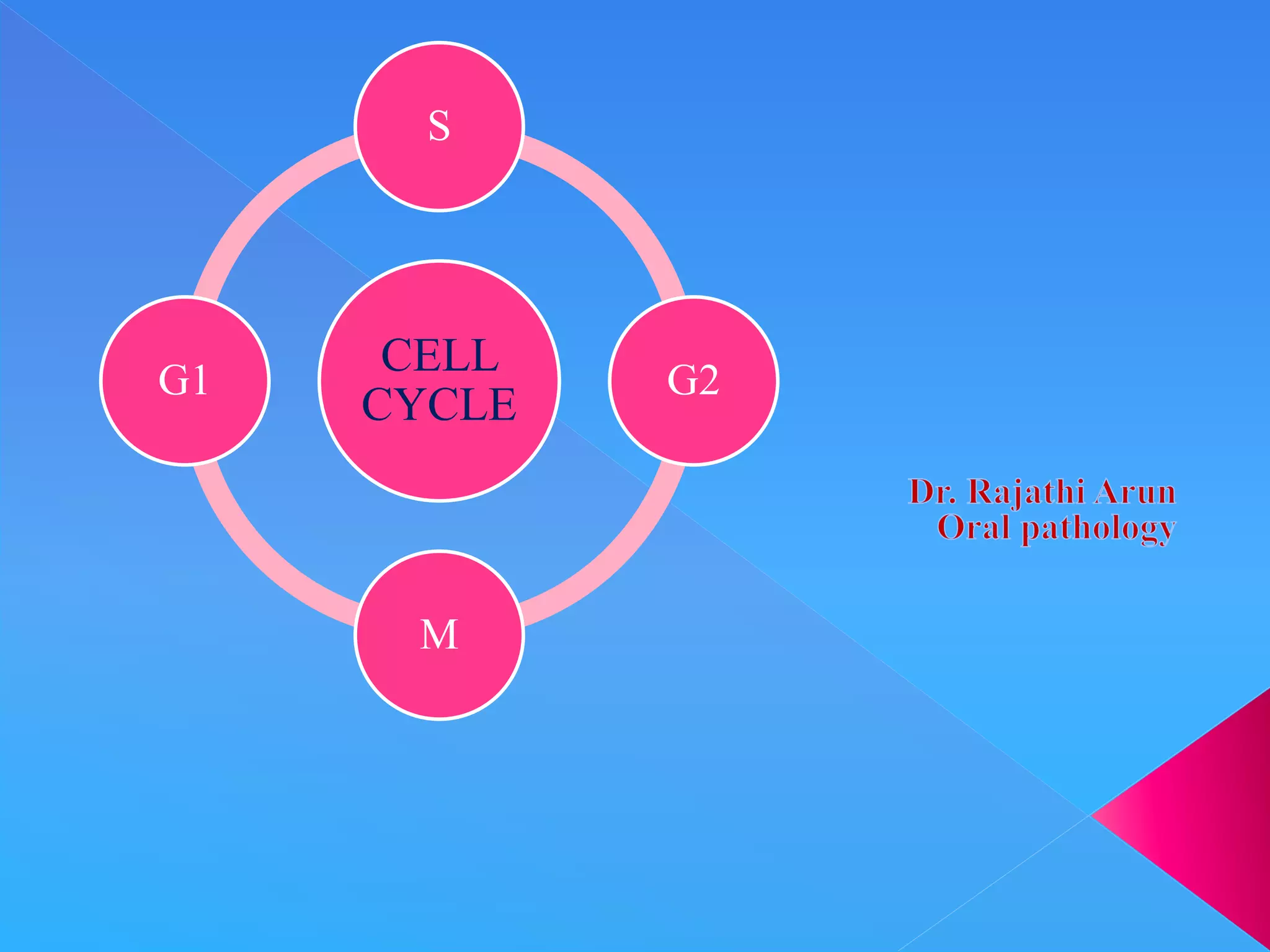
The document summarizes key aspects of the cell cycle. It describes the main phases (G1, S, G2, M), checkpoints that regulate progression, cyclins and CDKs that drive the cycle, and tissue types classified by their proliferative activity (continuously dividing, quiescent, nondividing). The cell cycle is highly regulated to ensure DNA replication fidelity and proper chromosome segregation through cyclin/CDK activity and checkpoint controls.