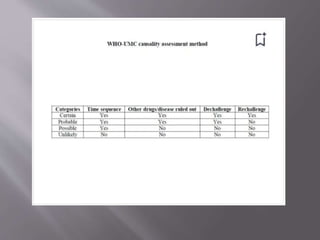
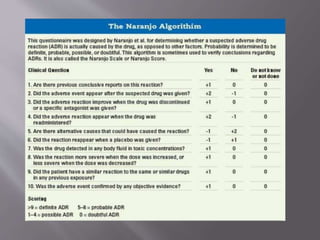
The document discusses various aspects of pharmacovigilance, including premarketing safety evaluation, post marketing surveillance, and methodological approaches for assessing adverse drug reactions (ADRs). It outlines different phases of human studies and the importance of reporting and documenting suspected ADRs through spontaneous reports and clinical studies. The document emphasizes training healthcare professionals and using standardized surveys to collect ADR information, alongside various causality assessment methods to determine the relationship between drug treatment and adverse events.