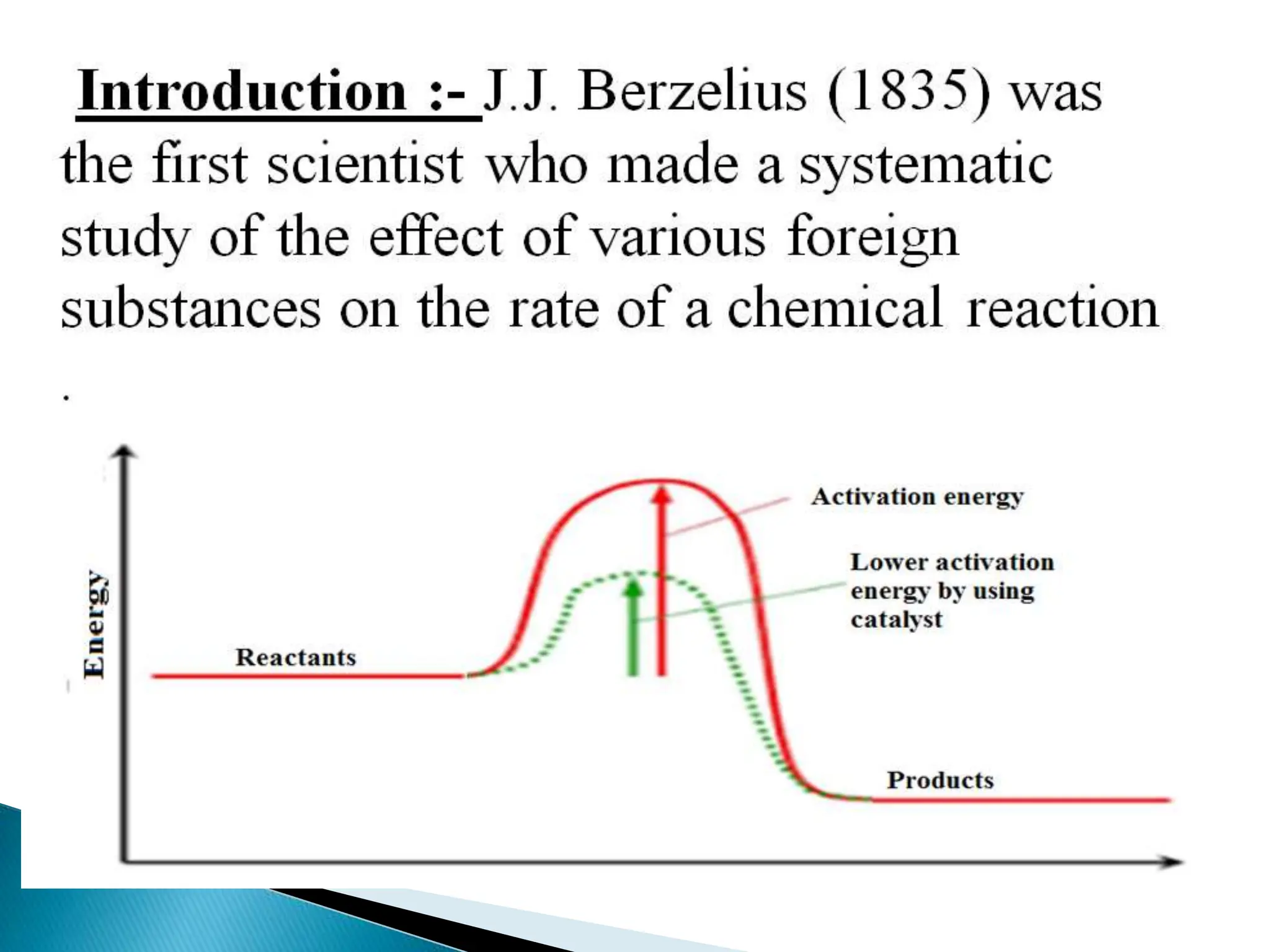
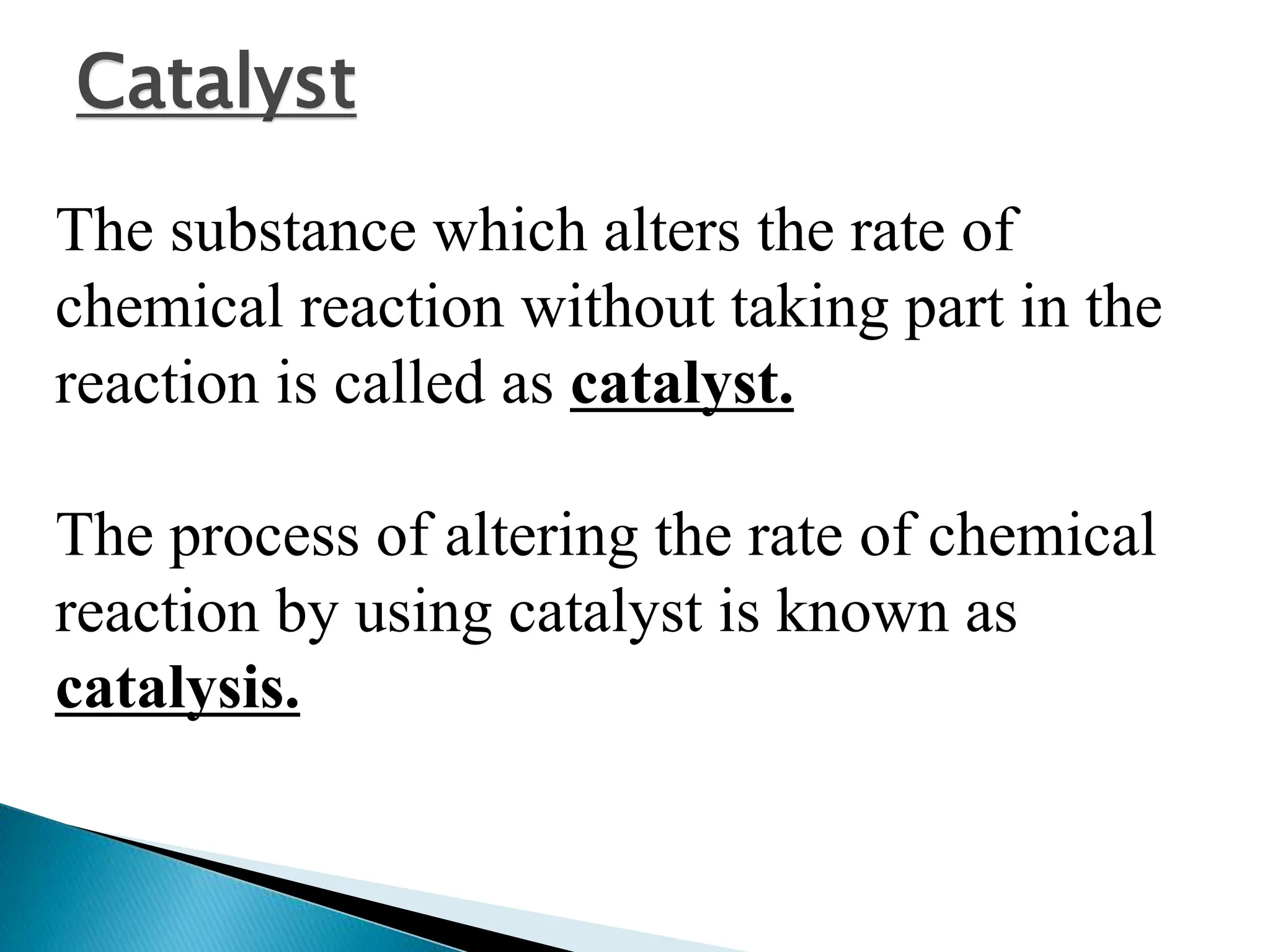
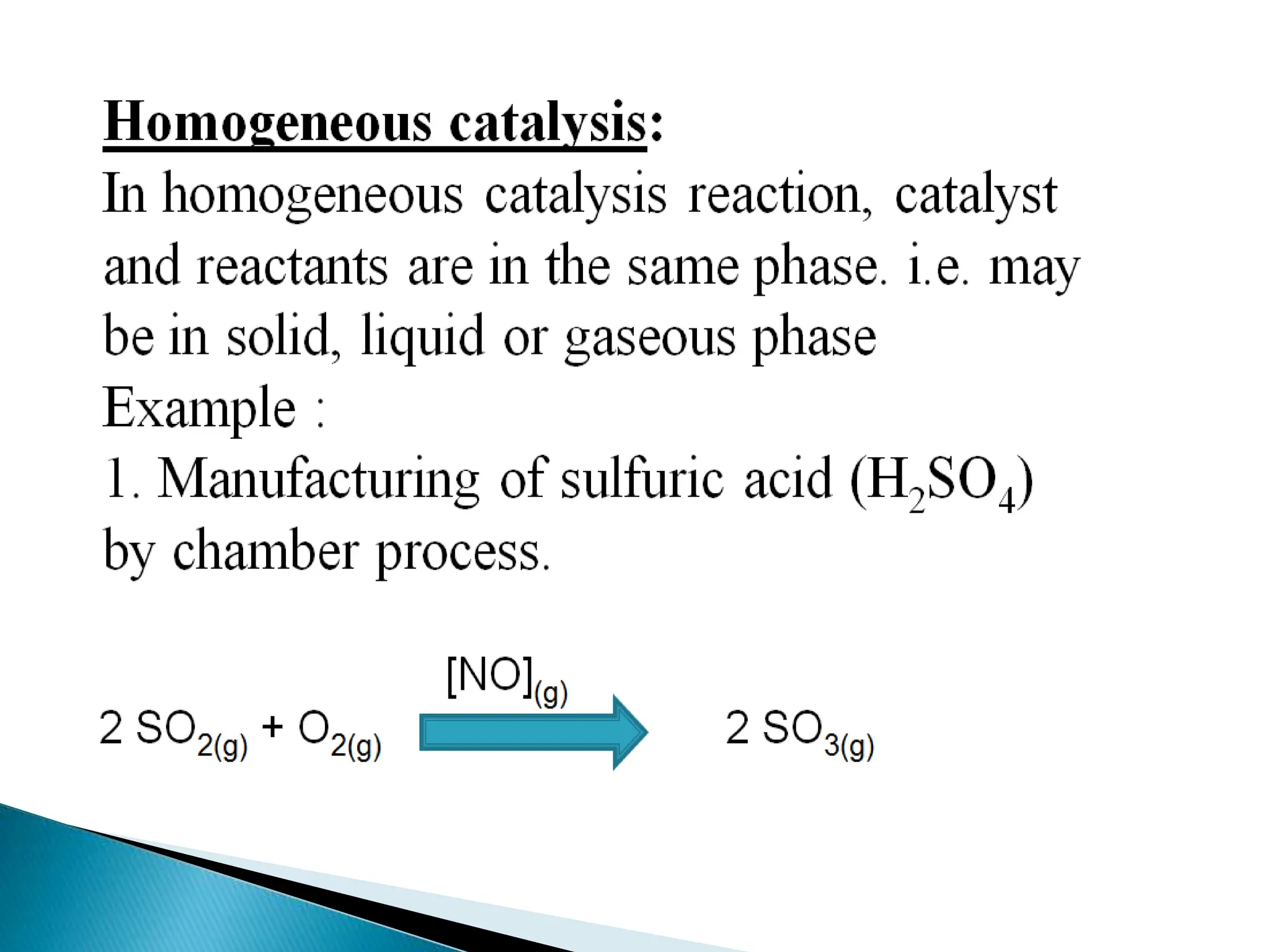
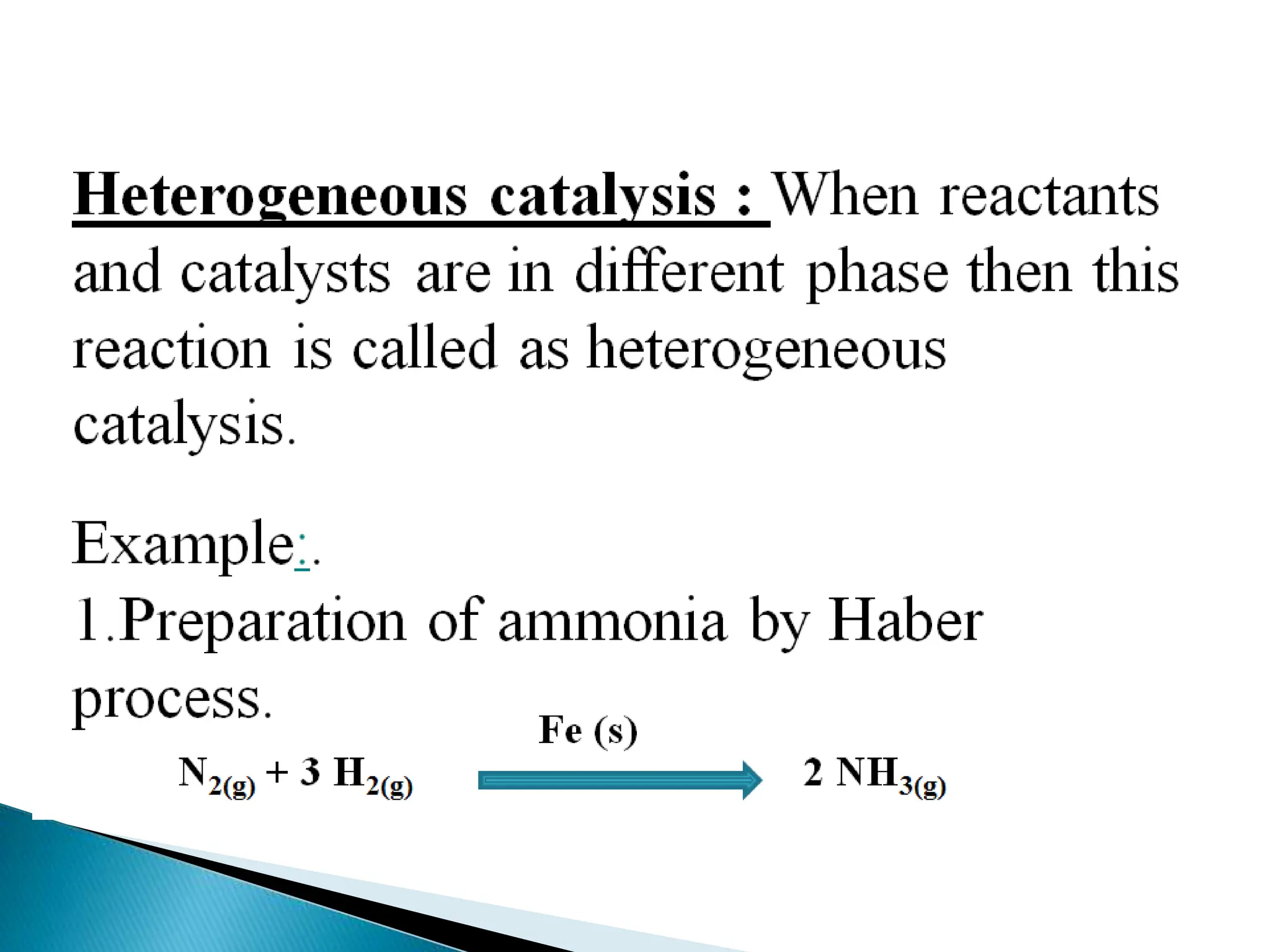
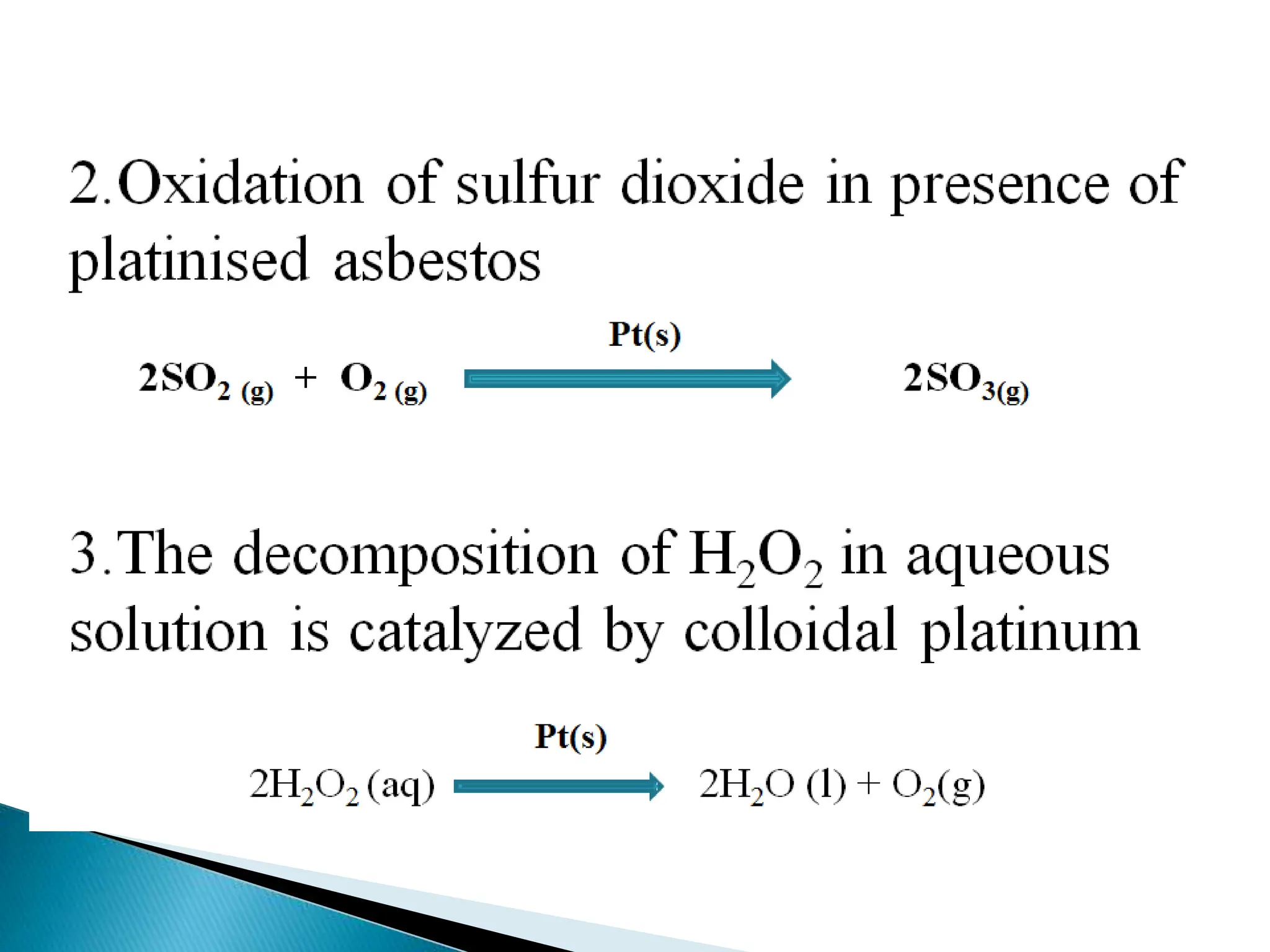
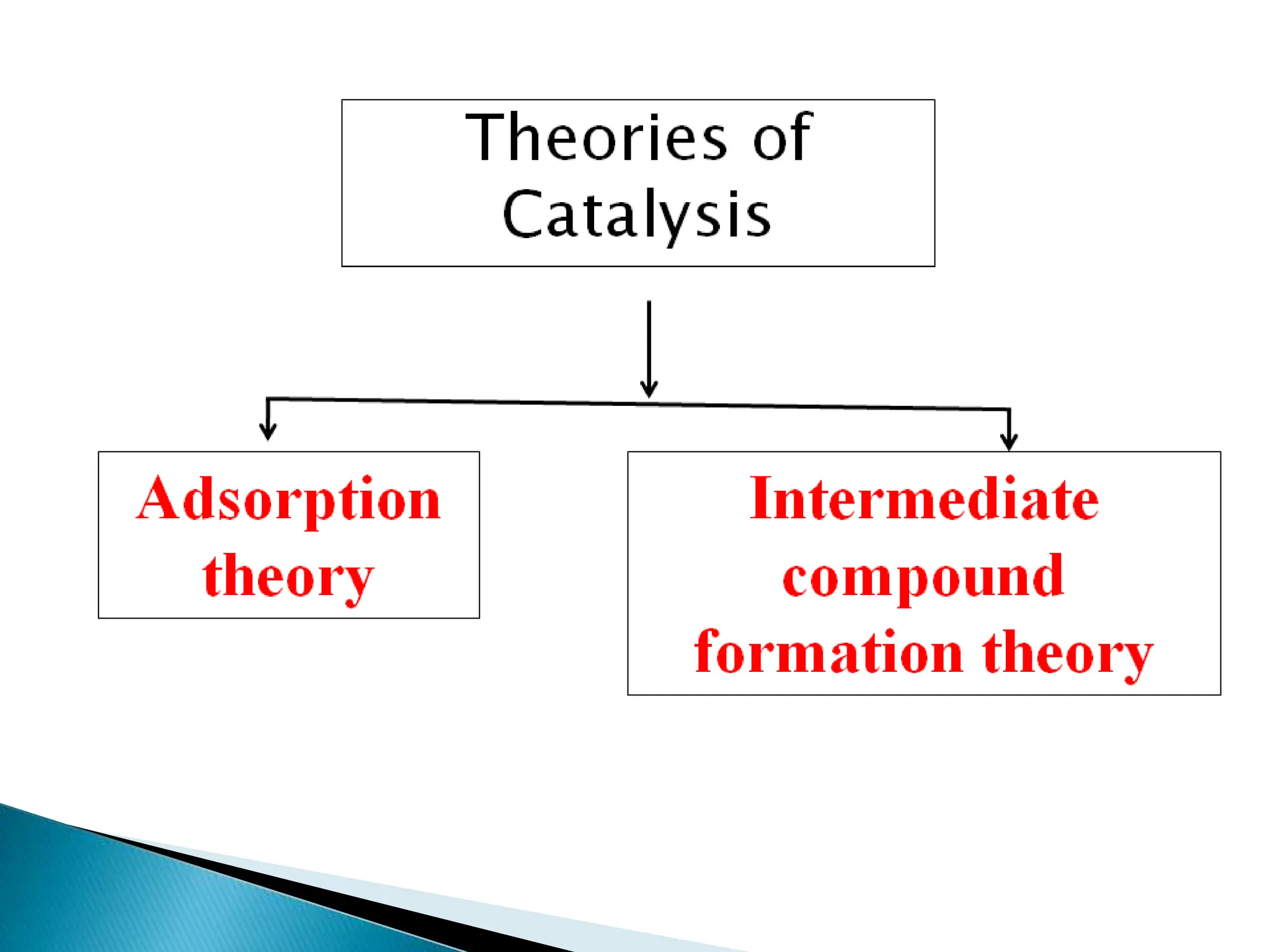
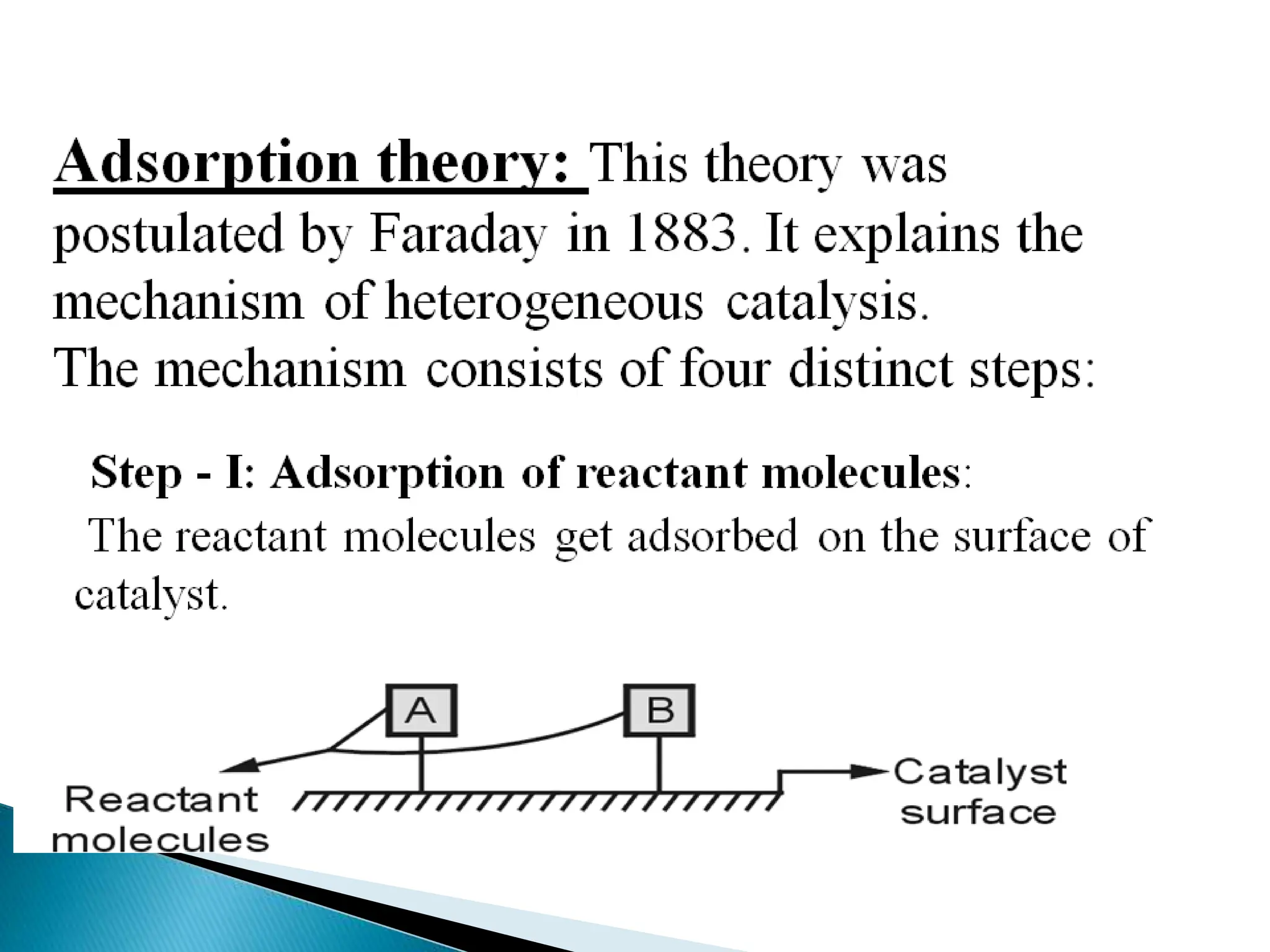
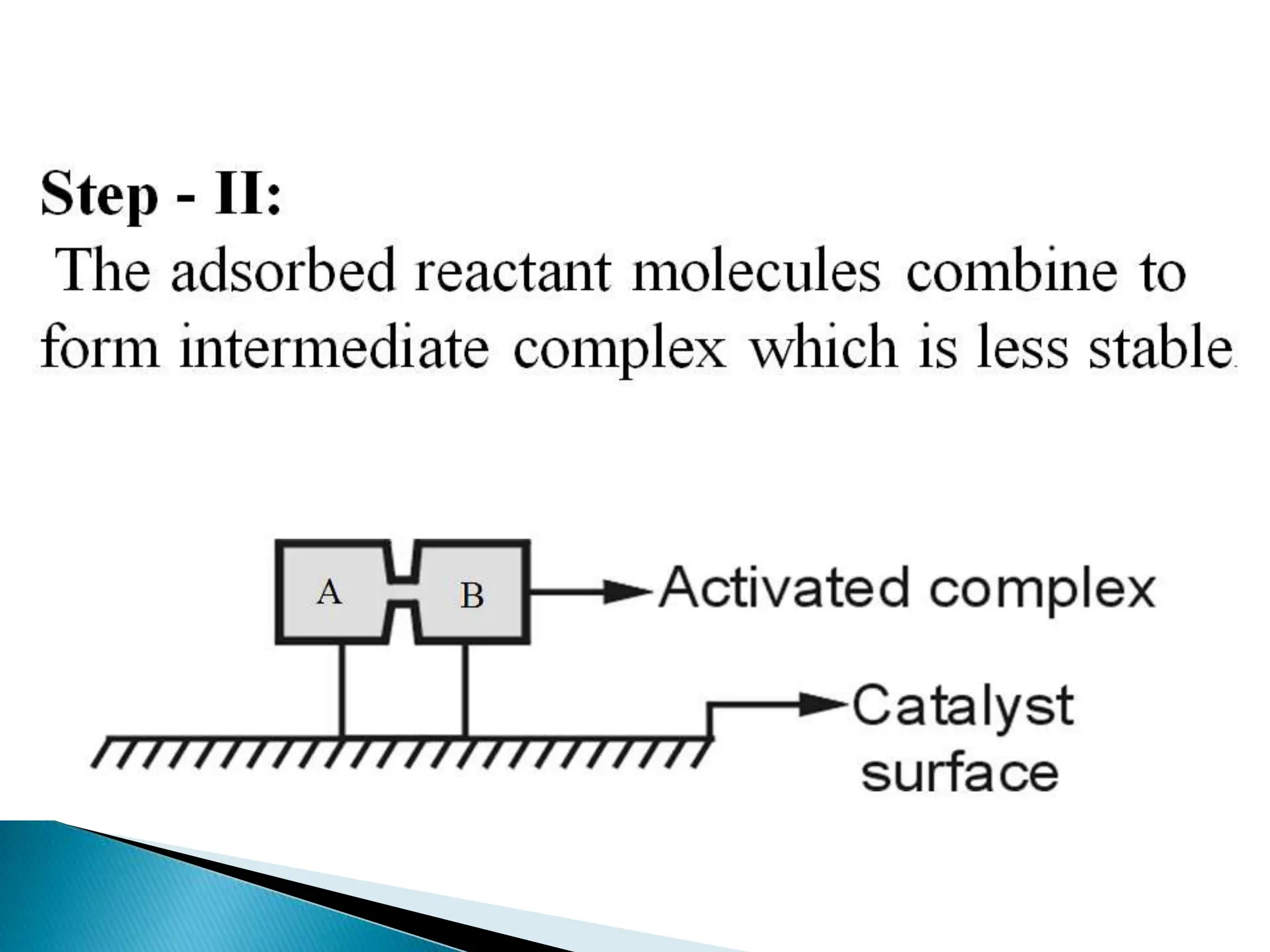
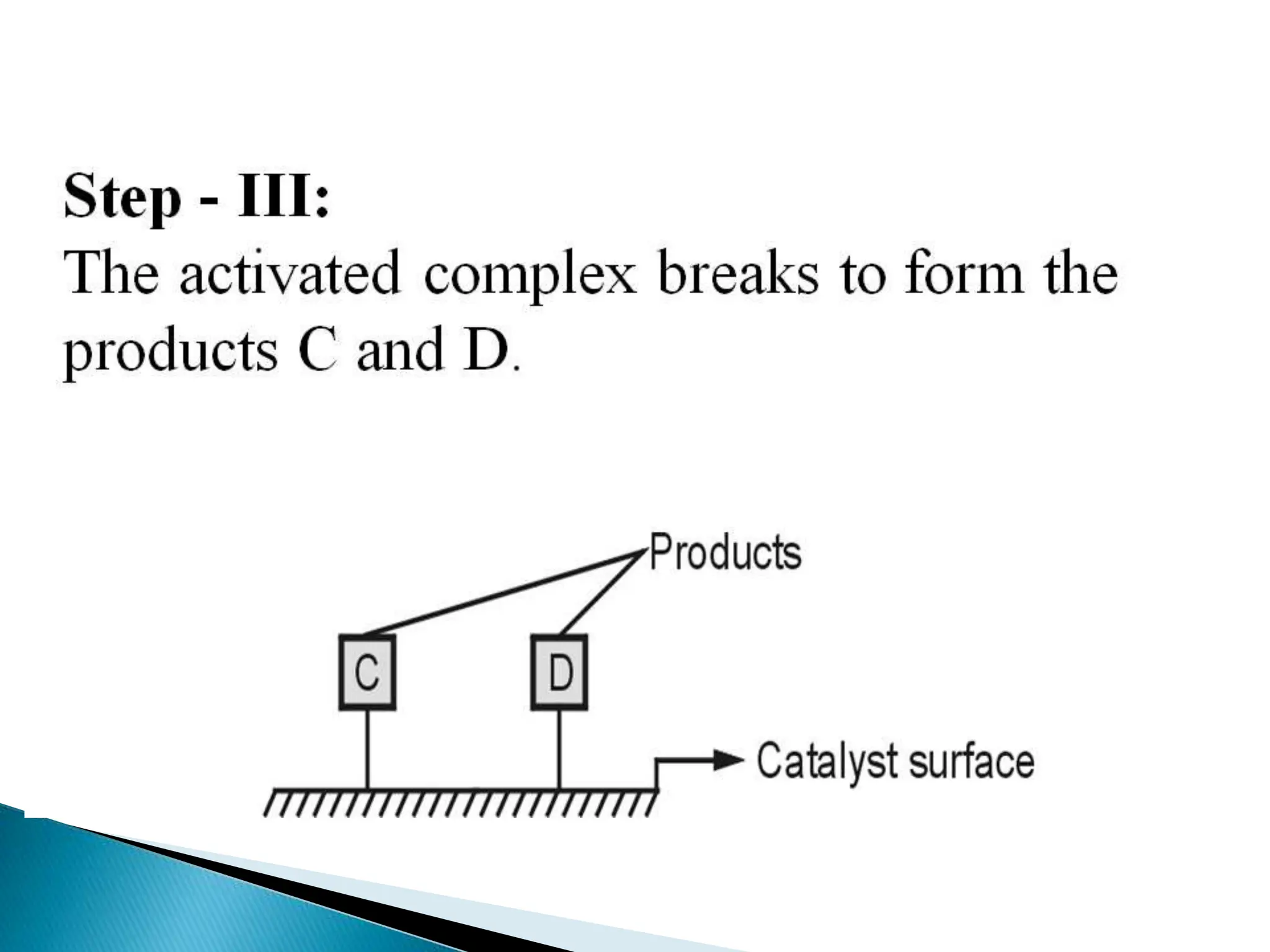
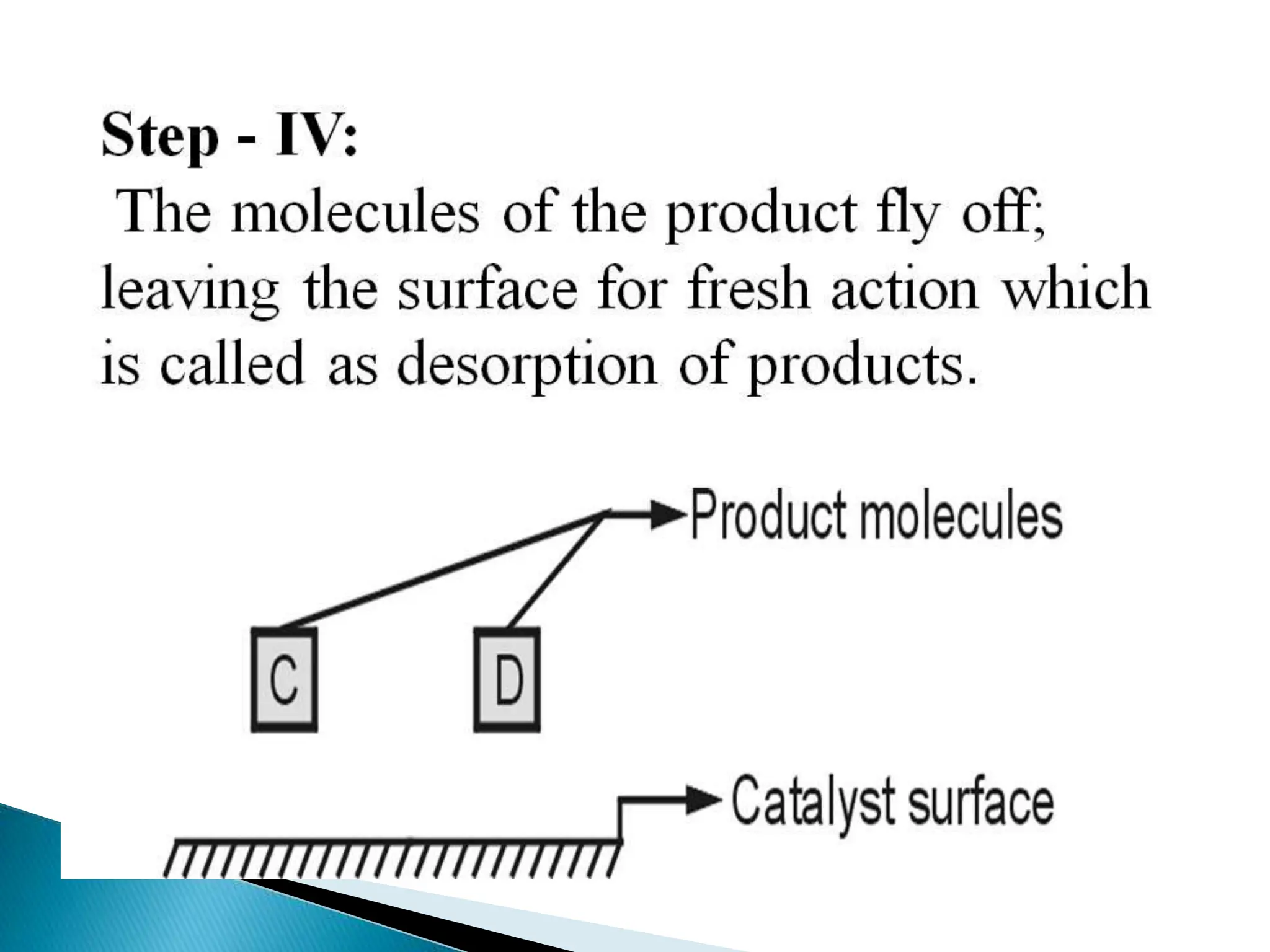
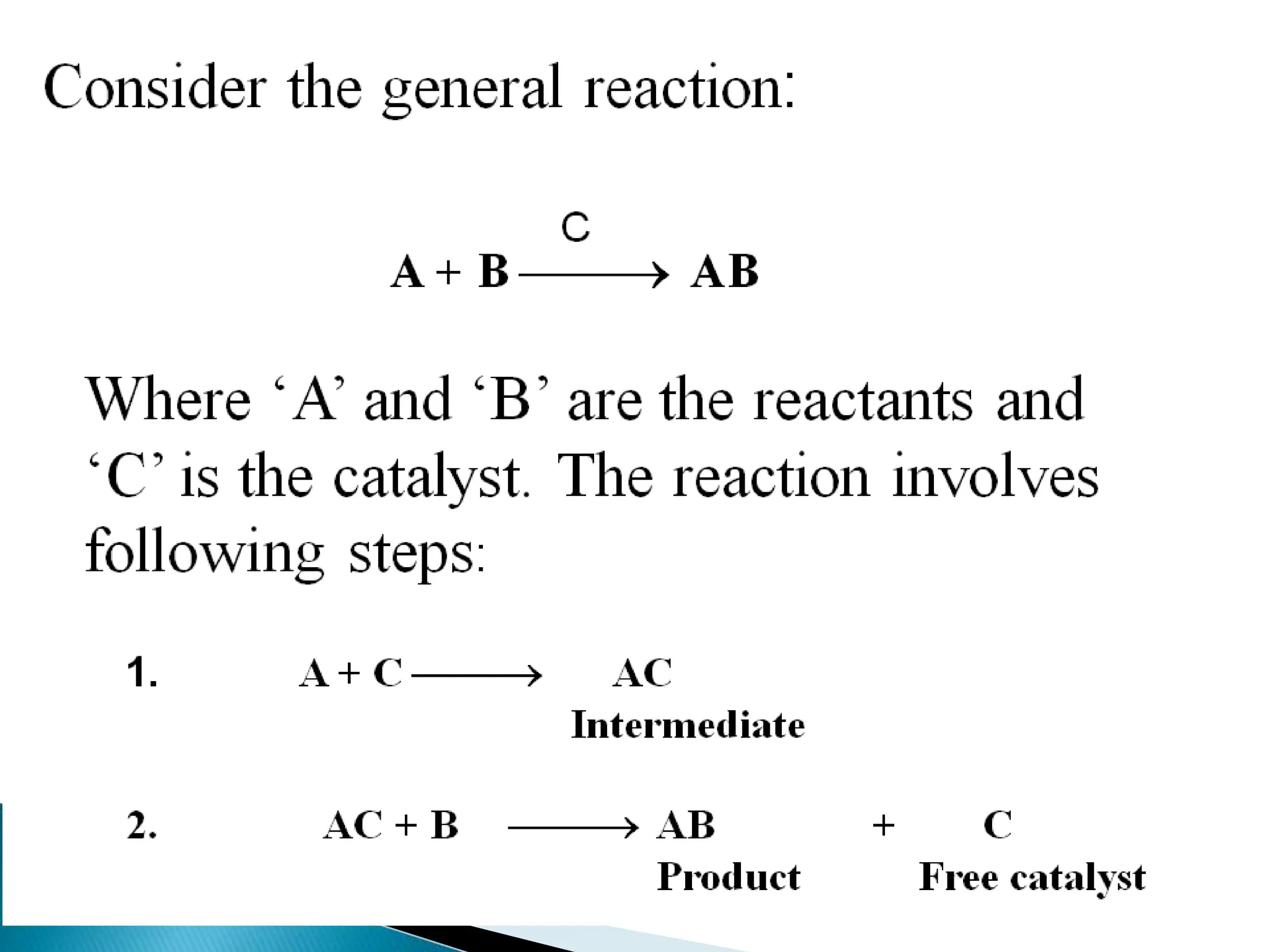
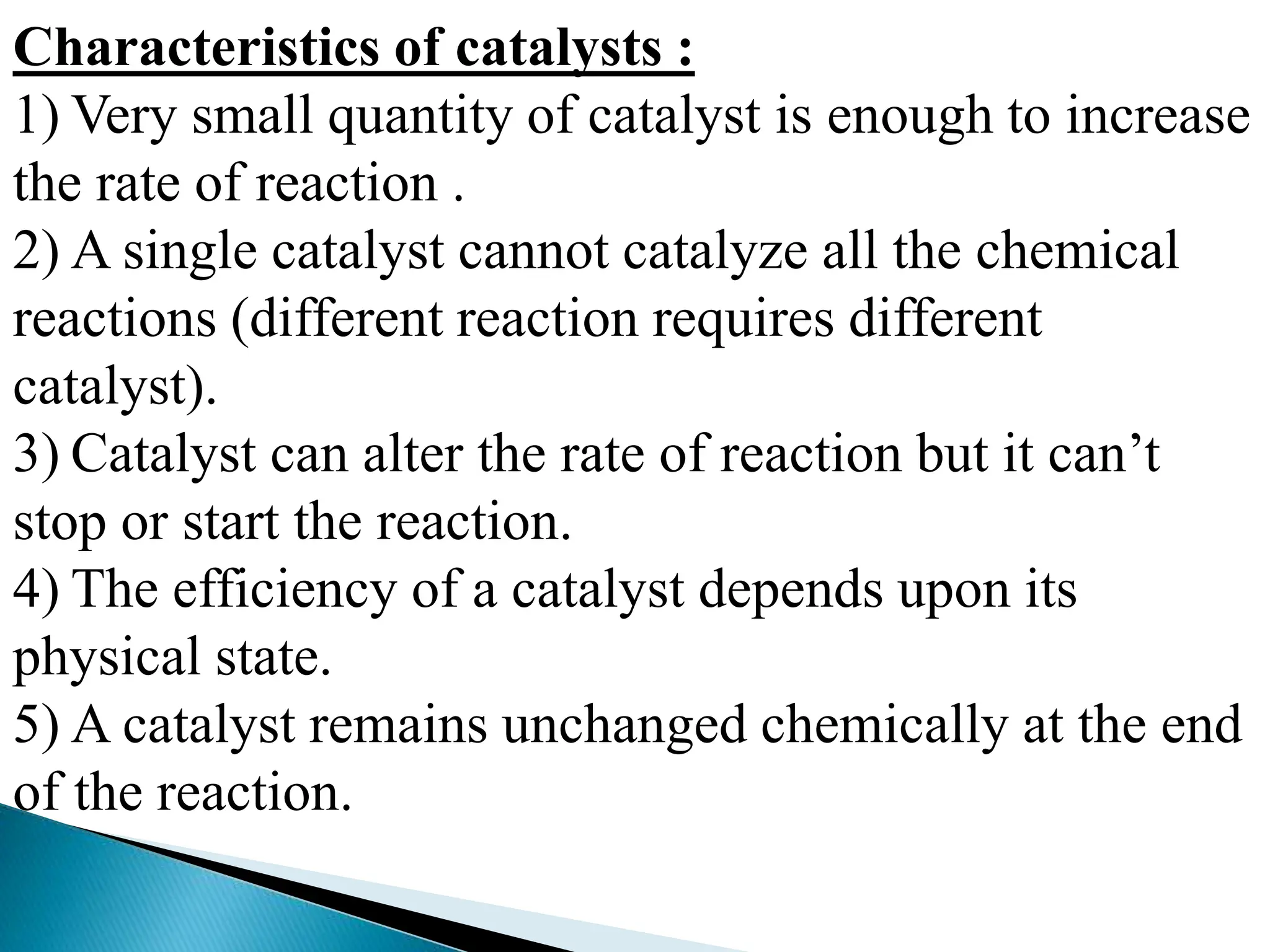
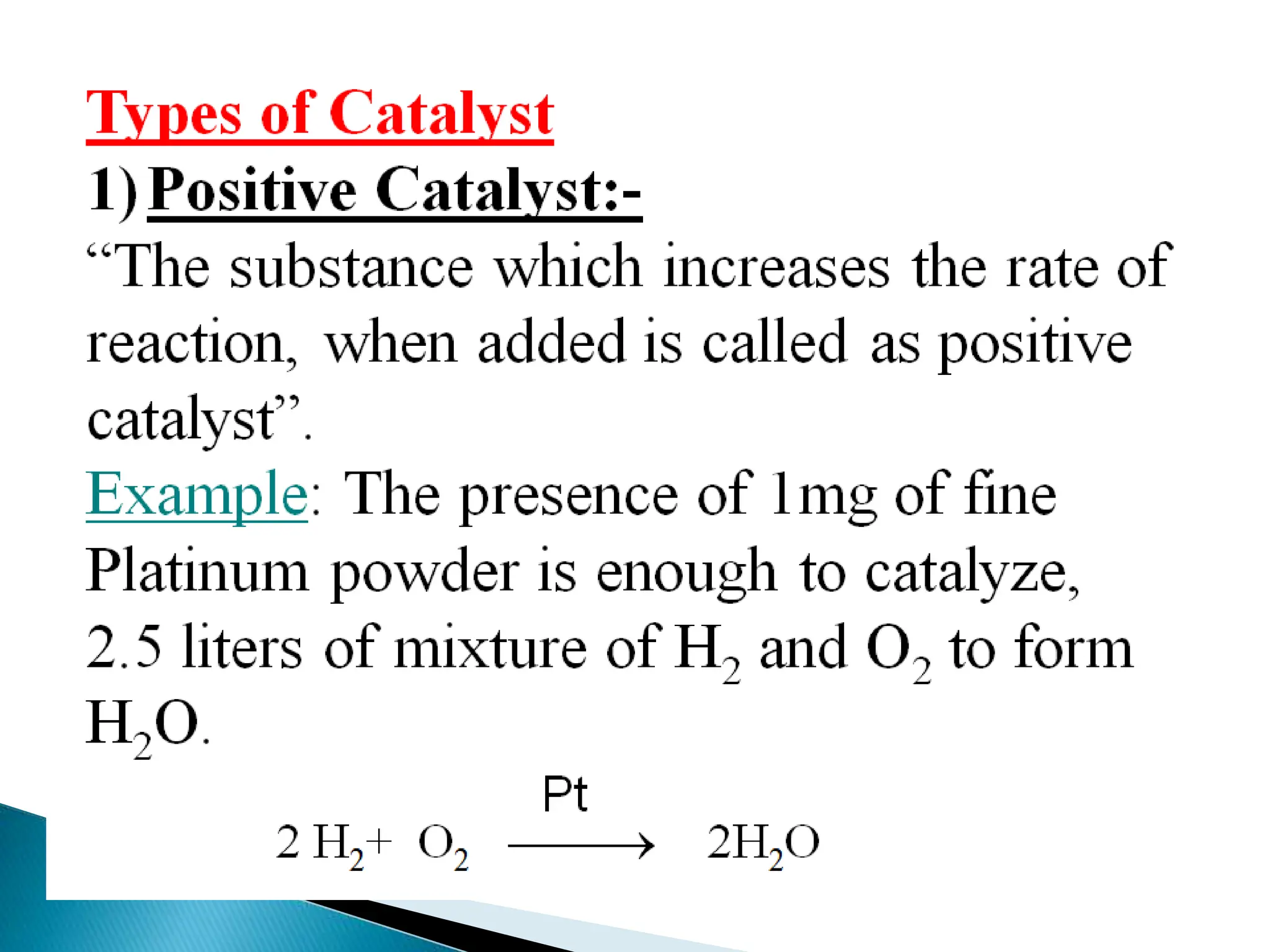
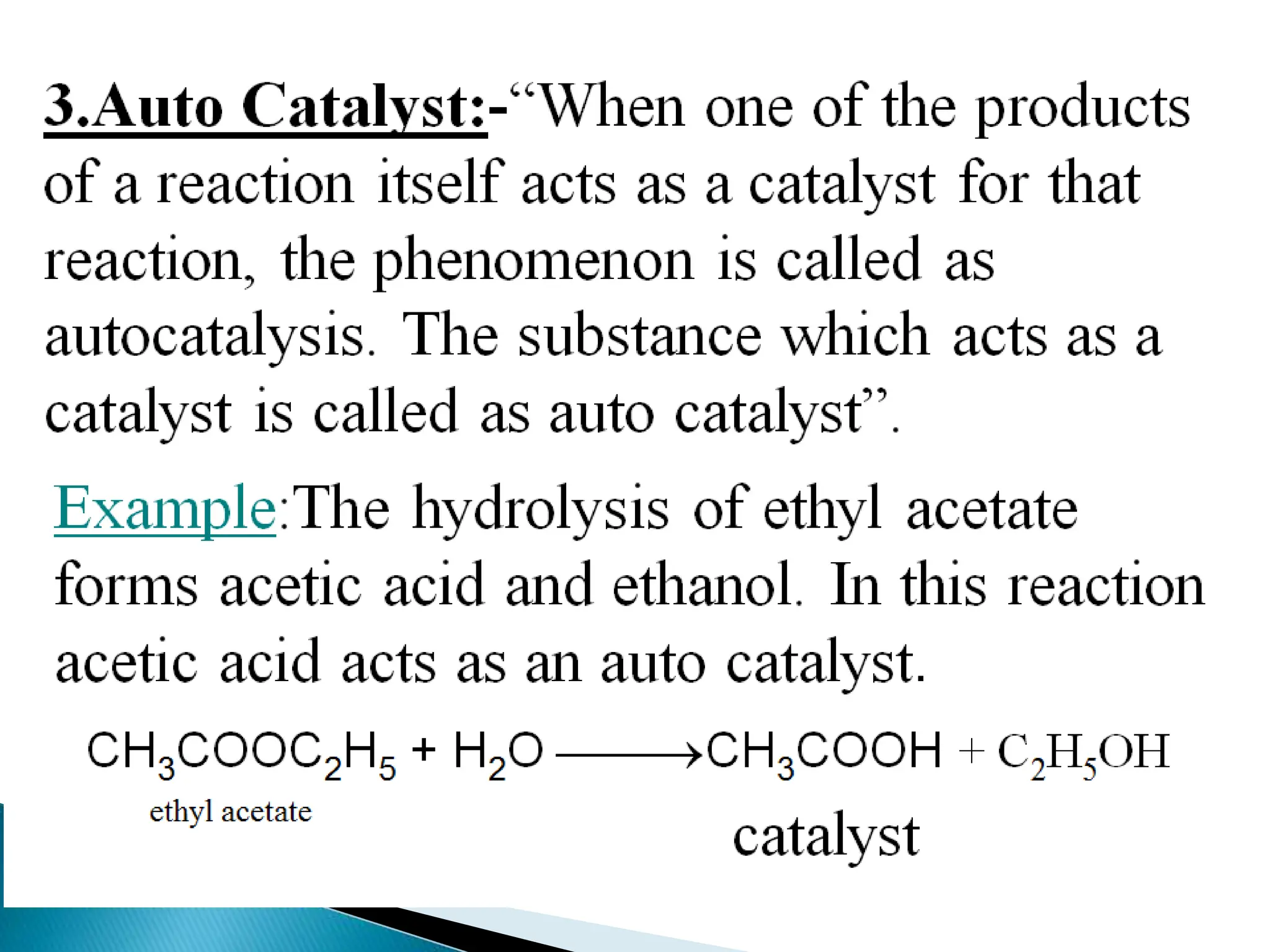
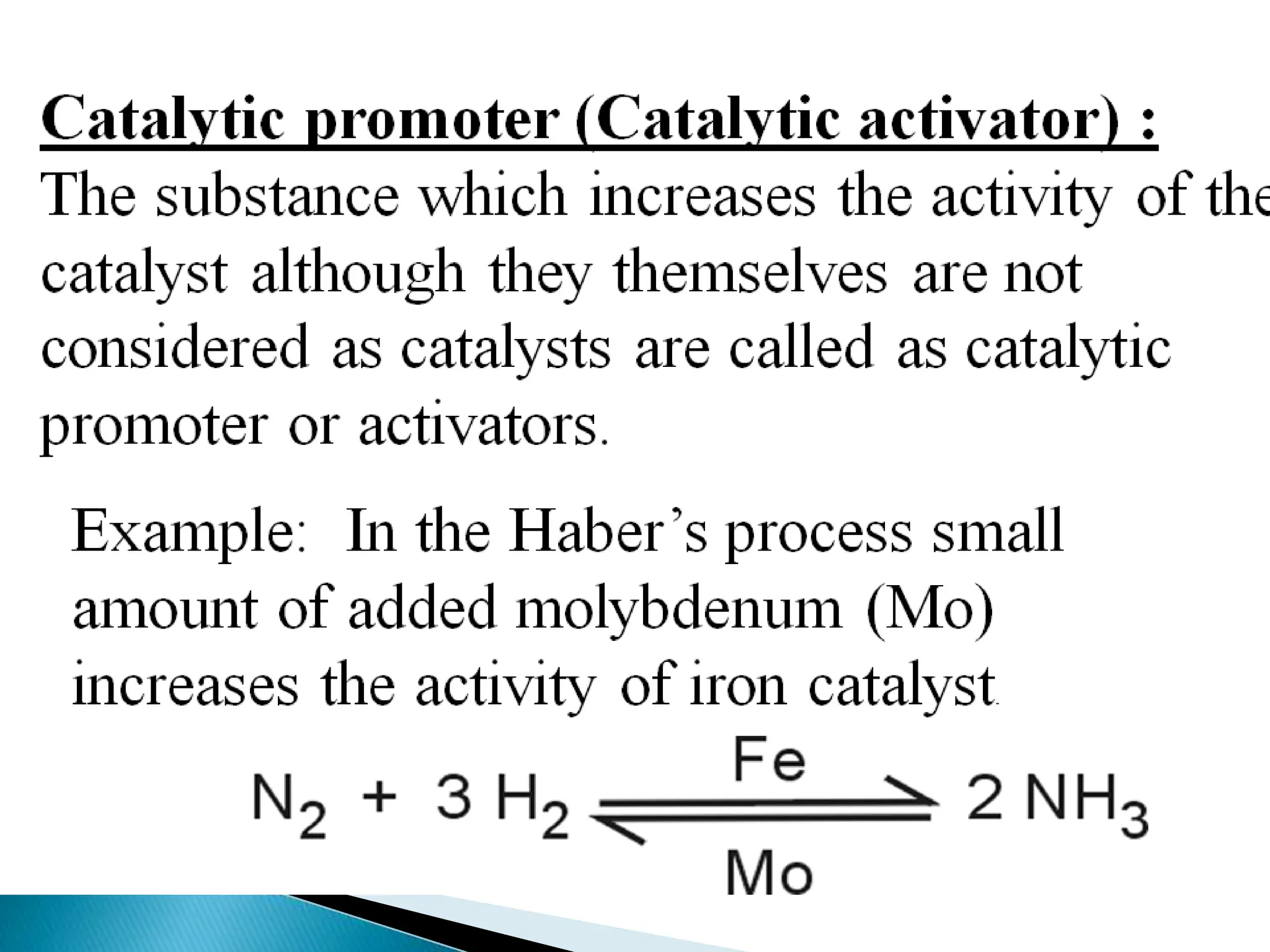
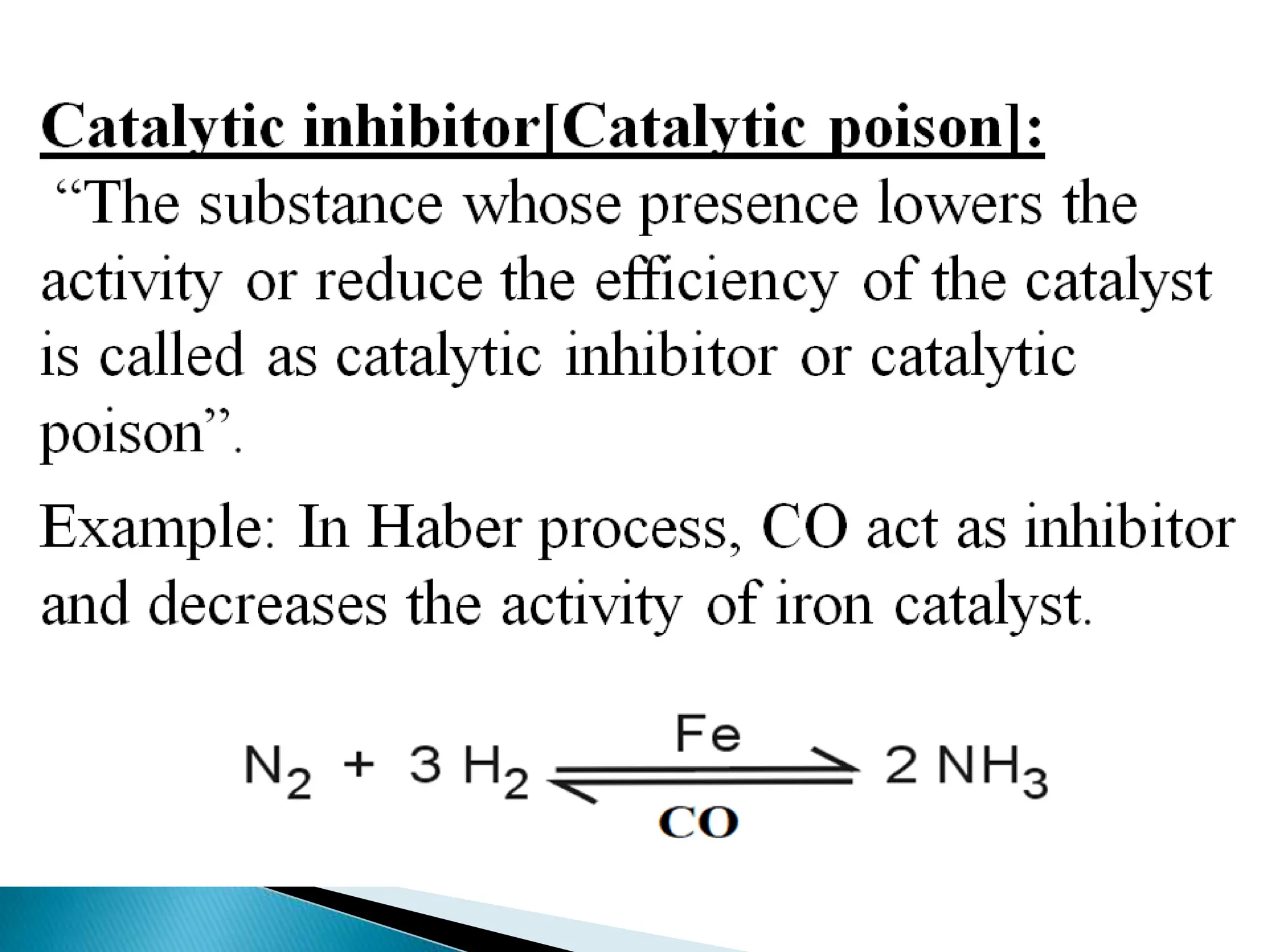
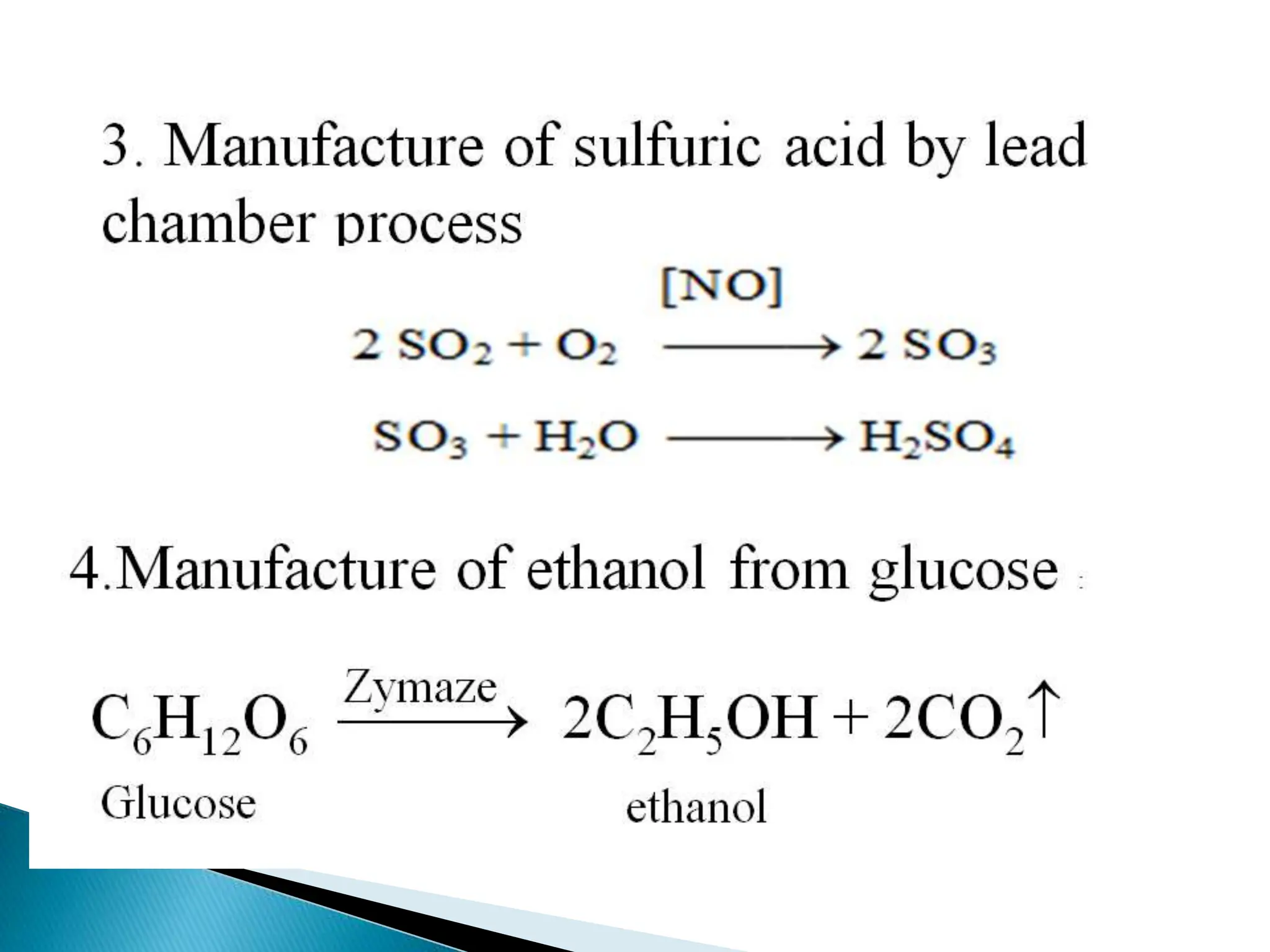
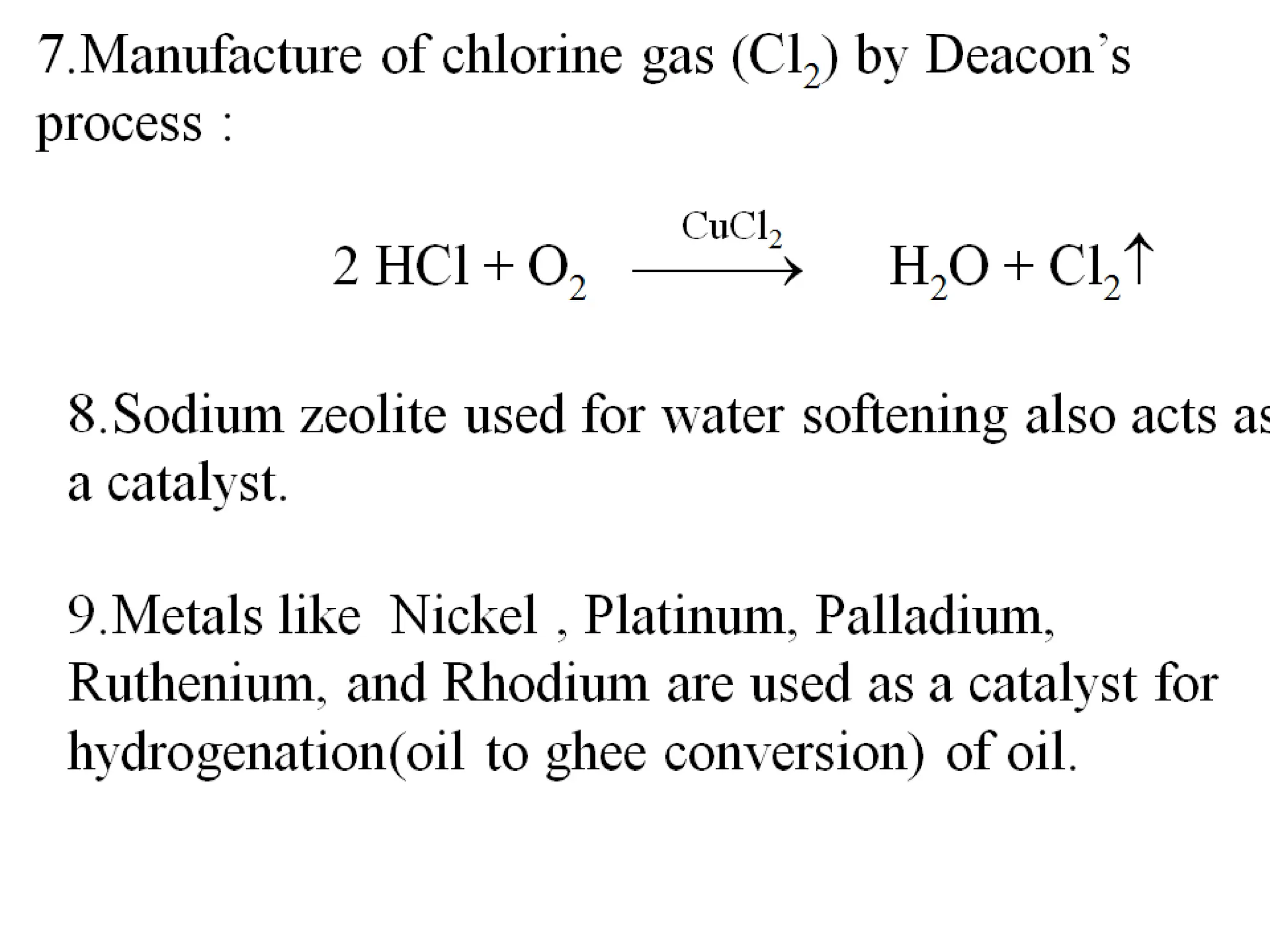
The document discusses catalysts and catalysis. It defines a catalyst as a substance that increases the rate of a chemical reaction without being consumed. Catalysis is defined as the process by which catalysts increase reaction rates. One theory provided is that catalysts form intermediate compounds with reactants that are more reactive. Characteristics of catalysts mentioned are that small quantities are effective, different catalysts are needed for different reactions, and catalysts remain unchanged after reactions. Some industrial uses of catalysts and example catalysts are also listed.