This document discusses solid base catalysts as a green approach for chemical synthesis. It describes various types of solid bases including hydrotalcites, zeolites, and amines immobilized on silica. Hydrotalcites have basic sites in the interlayer space and can catalyze aldol condensations and ionone synthesis as a replacement for NaOH. Zeolites with exchanged alkali cations or loaded metal oxides also exhibit basic properties and can catalyze reactions like the Knoevenagel condensation. Immobilizing amines and ammonium groups on silica via grafting produces heterogeneous basic catalysts. Solid bases offer advantages over liquid bases like recyclability, easy separation, and generating less waste

![SOLID BASES
The solids having the sites which serve as a Bronsted base and/or
Lewis base are called SOLID BASES. These are used in place of liquid
bases like NaOH, KOH etc.
AH B
- A
- BH
A B A B
A] Bronsted-Lowery concept of conjugate acid -base pair-
B] LEWIS -Base concept -
lewis base
BASE Conjugate-acid
The solids having the sites which serve as a Bronsted base and/or
Lewis base are called SOLID BASES.](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-3-320.jpg)
![With Proton Abstraction- On the surface of solid bases, there are
specific sites or centers, which function as a base. Basic
sites(centers) abstract protons from the reactant molecules(AH) to
form a carbanion(A-).Here the basic site act as a bronsted base.
AH B
-
A
- BH
BASE Conjugate-acid
Activation of reactants on SOLID BASES- It occurs by
two ways-
1]
2] Without Proton Abstraction- Reactants such as ketones and
aldehydes are often activated by bases without proton transfer, here
the basic sites B- act as lewis base. This kind of interaction is important
in many base-catalysed reactions such as Aldol-
Condensations,Knoevenagel condensation and hydrogen transfer
reactions.
C O B
- H3C
B
O-](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-4-320.jpg)

![PhCH2CN CH3I NaOH NaI H2O
1] CONVENTIONAL METHOD - The Methylation of Phenylacetonitrile with
Methyl Iodide proceeds in the presence of Bases under a Phase-Transfer
Condition.
SN2
REACTION
PhCH(CH3)CN
2] GREEN APPROACH - The methylation of Phenylacetonitrile by using methanol
or Dimethyl carbonate as the methylating agent and alkali-exchanged zeolites as
catalysts. The reaction can be performed in vapour phase.
PhCH2CN CH3OH
ZEOLITES
Phenylacetonitrile Base(needed in
stochiometric amounts)
Toxic
reagent
Inorganic salt(disposing
problem)
PhCH(CH3)CN H2O
Phenylacetonitrile
SN2 REACTION
COMPARISON B/W CONVENTIONAL
AND GREEN METHODS -
Green by product](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-6-320.jpg)

![O O
NaOH
CH2
CH3
H
O O
O
O
-
OH-
-
O
H H
O
O H
α
β
H H
-OH-
NaOH
Δ
O
α
β
H
H
H
O
-H20
O
β
H
α
Acetone
Enolate
3] Protonation
4] Dehydration α,β-Unsaturated ketone
1] Abstraction of proton 2] Enolate attack
General Mechanism Of
ALDOL-CONDENSATION-](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-8-320.jpg)


![1
2
ALDOL CONDENSATION BY HYDROTALCITE AS A
BASE IN PLACE NaOH
CH2OH
2-Ethylhexanol
[Methylisobutylketone]
(MIBK) and 2-ethylhexanol, the precursor of the PVC
plasticizer, and are used in Paints.](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-11-320.jpg)

![O
OH
-
Pseudoionone
Acetone
Citral
CH CHCOCH3
CH CHCOCH3
OR
CH CHCOCH3
β - ionone
H+
+
Conventional Method - IONONE
SYNTHESIS BY ALDOL-CONDENSATION
[Aldol Condensation]
60% conv.
80% selectivity
1
2
3
4
α - ionone
Me
O
Me
Me Me
Me
Me
CHCCH3
O
Me
Me
Me
CH
Me
Me Me
H
H
α
β
α
β
α
β
CH CHCOCH3
CYCLIZATION](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-13-320.jpg)

![O CH CHCOCH3
Pseudoionone
Acetone
Citral
CH CHCOCH3
OR
CH CHCOCH3
α - ionone β - ionone
[Aldol Condensation]
>95% conv.
70% selectivity
HYDROTALCITE
HYDROTALCITE method for the
synthesis of IONONES
1
2
Ionones are used in perfume
industry.
CHO
α
β
α
β](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-15-320.jpg)
![2) Zeolites as Solid Bases –
Zeolites are crystalline aluminosilicates with pores of molecular dimensions.
General formula = M[(AlO2) x(SiO2) y].MH2O
AlO2and SiO2 are fundamental units sharing oxygen ions to form tetrahedral Al04
and SiO4 building blocks for zeolite unit cell.](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-16-320.jpg)






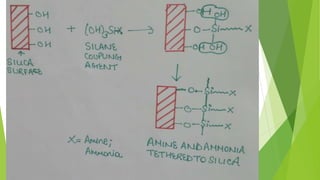



![Catalysis by solid bases [recovered] [autosaved]](https://image.slidesharecdn.com/catalysisbysolidbasesrecoveredautosaved-210612140910/85/Catalysis-by-solid-bases-recovered-autosaved-27-320.jpg)