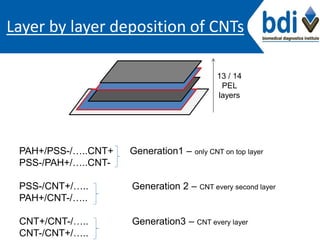
This project aims to functionalize carbon nanotube (CNT) films with antibodies to improve their biocompatibility for use as cell culture platforms and biomedical applications. CNT films will be prepared using layer-by-layer deposition with polyelectrolytes and functionalized with antibodies via covalent attachment or physical adsorption. The films will be characterized using Raman spectroscopy, fluorescence microscopy, and electrochemical analysis. Cell culture studies will investigate the ability of antibody-modified CNT films to support cell adhesion and proliferation. Overall, the project seeks to develop novel electrically conducting biomaterials for applications such as tissue regeneration.