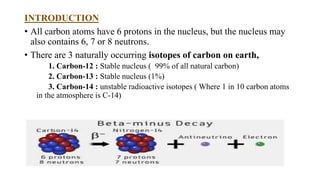
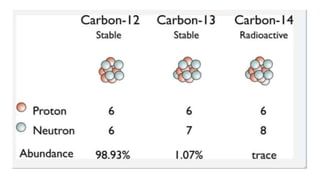
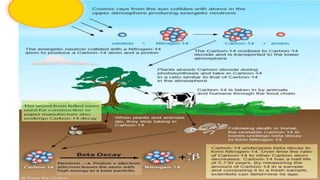
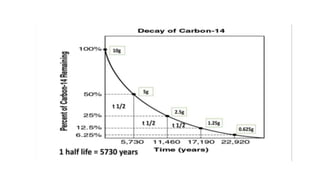
Carbon dating uses the radioactive isotope carbon-14 to determine the age of organic materials. The document outlines the history, principles, applications, and limitations of carbon dating. Specifically, it notes that carbon-14 is formed in the atmosphere and incorporated into living things, but after death the amount of carbon-14 decays at a known rate, allowing the age of a sample to be estimated by measuring its remaining carbon-14. Examples are given of carbon dating being used to determine the ages of artifacts such as ancient manuscripts, cave paintings, and archaeological sites.