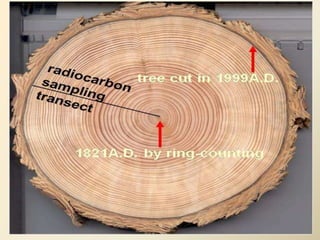
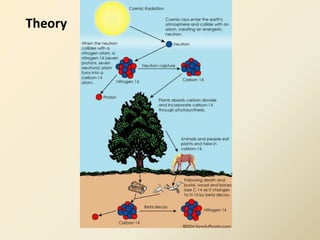
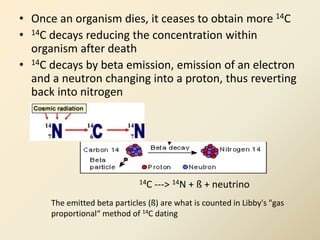
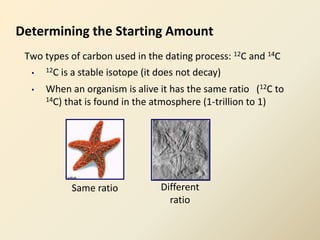
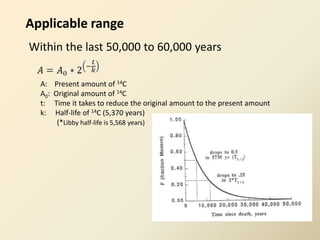
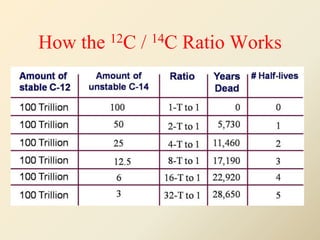
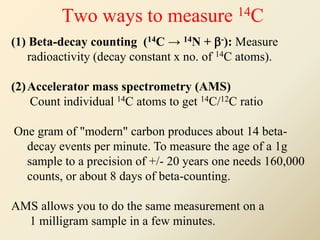
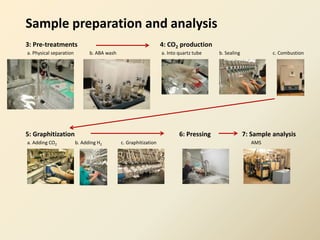
Radiocarbon dating is a method used to determine the age of organic material by measuring the amount of radioactive carbon-14 remaining in the sample. It relies on two key facts: 1) all living things absorb carbon from the atmosphere in a stable carbon-12 to carbon-14 ratio, and 2) carbon-14 decays after death at a known rate. By measuring the carbon-14 level in a sample and calculating how many half-lives it would take to decay to that level from the original ratio, the age can be estimated. However, calibration is required to convert radiocarbon years to calendar years due to past fluctuations in atmospheric carbon levels. Samples must also be properly collected and pre-treated to avoid contamination.