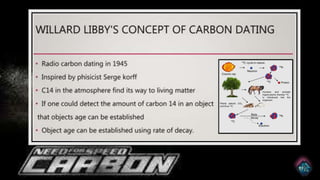
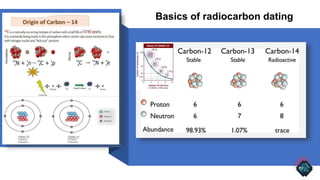
Radiocarbon dating, or carbon-14 dating, is a method used to determine the age of organic materials through the decay of carbon-14, providing significant insights into archaeology, geology, and environmental studies. Despite its effectiveness, the technique faces challenges such as sample contamination, calibration curve variability, and limitations with older samples. Technological advancements like accelerator mass spectrometry have enhanced its precision and applicability, promising future developments in research and understanding of human history.