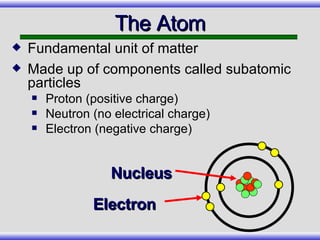
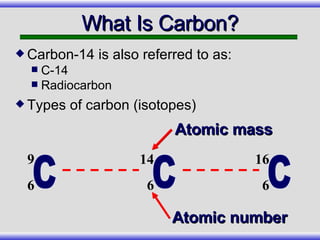
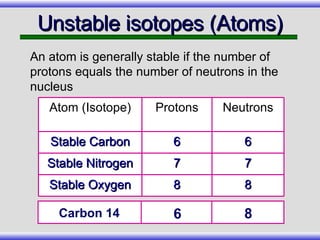
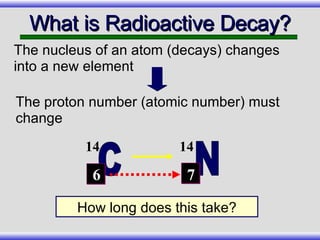
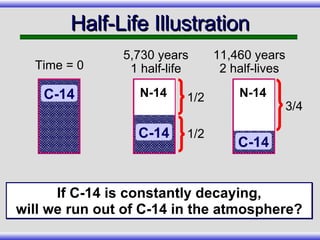
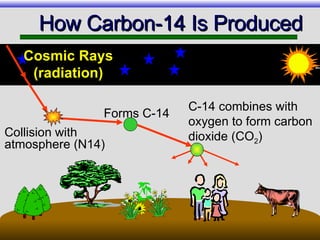
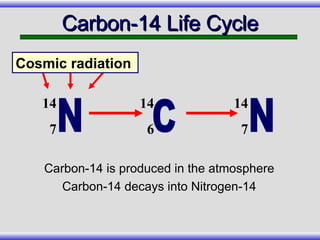
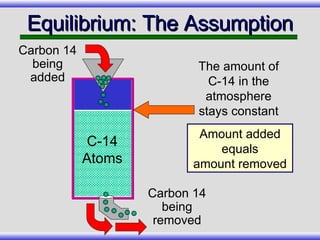
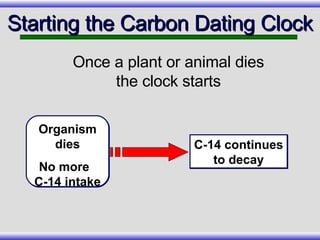
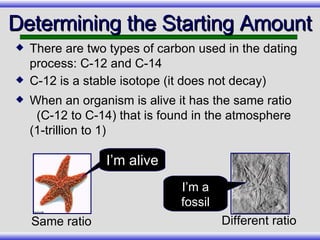
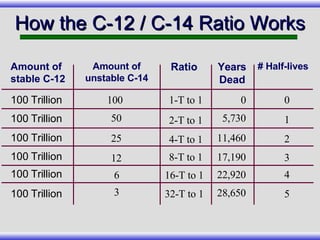
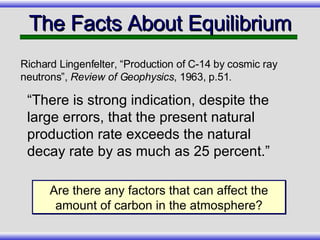
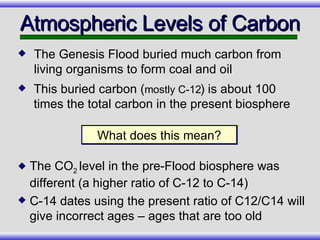
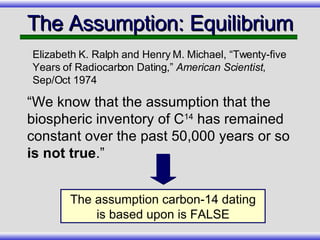
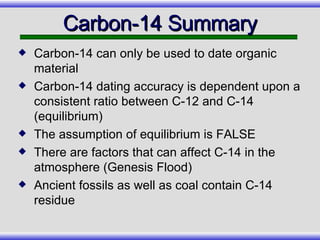
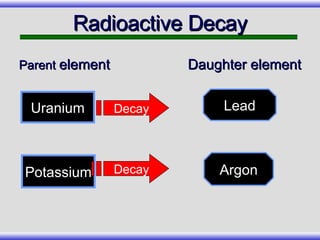
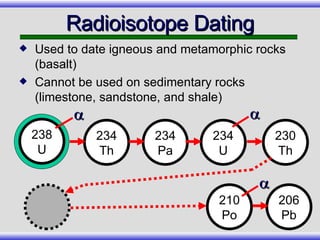
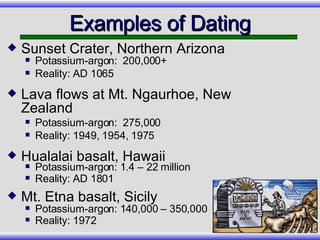
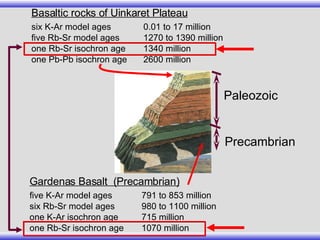
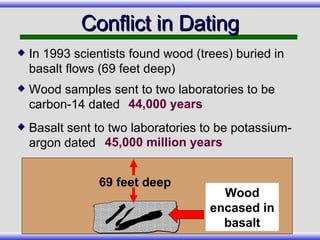
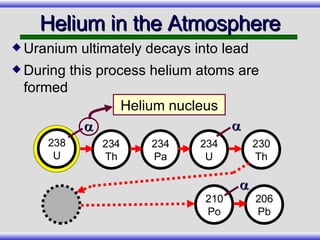
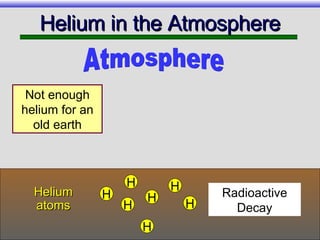
The document provides an overview of carbon-14 dating, detailing its principles, limitations, and assumptions, particularly the need for a consistent carbon-12 to carbon-14 ratio. It argues that various factors, including atmospheric changes and events like the Genesis flood, could compromise the accuracy of carbon dating and suggests that ancient fossils and coal contain detectable levels of carbon-14, contradicting the notion of their extreme age. Additionally, it challenges the reliability of radioisotope dating methods by illustrating discrepancies in dating results and advocating for a young earth perspective based on recent scientific findings.