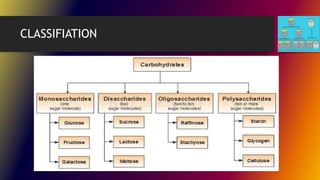
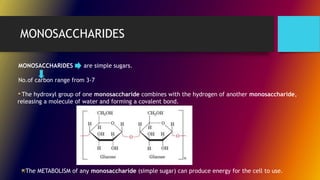
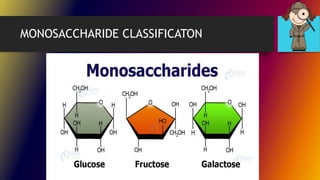
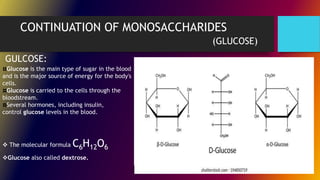
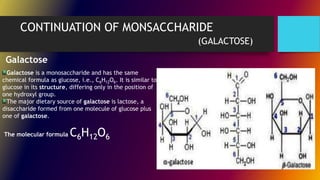
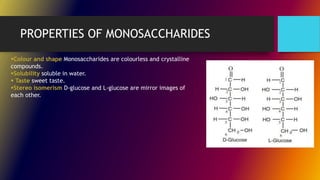
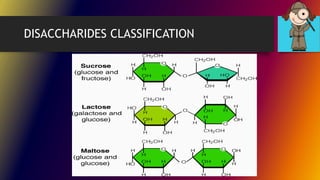
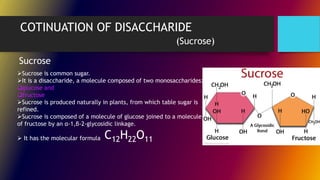
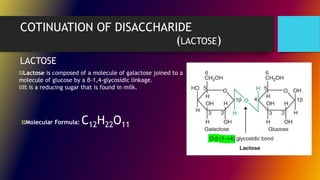
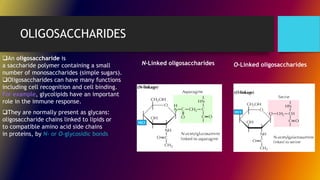
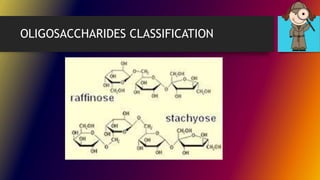
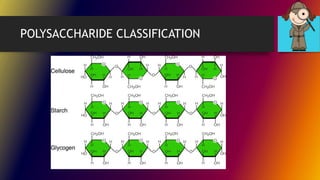
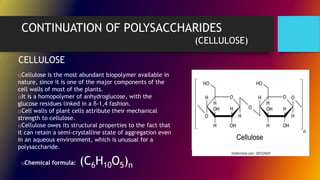
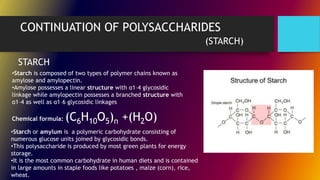
Carbohydrates can be classified into monomers, dimers, and polymers. Monosaccharides are simple sugars with 3-7 carbons like glucose, fructose, and galactose. Disaccharides are double sugars formed when two monosaccharides bond, examples include sucrose, lactose, and maltose. Polysaccharides are long chains of monosaccharides and include starch, cellulose, and glycogen. Carbohydrates serve important biological roles like energy storage, structure, and cell communication.