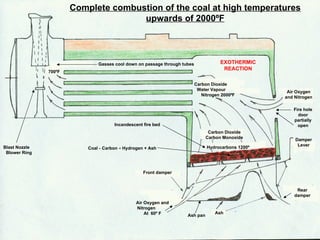
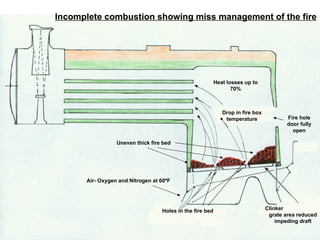
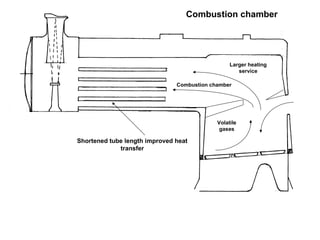
The document discusses coal combustion in boilers. It describes the different types of coal and their properties, including anthracite, dry Welsh steam coal, and bituminous coal. It also covers the combustion process, including the roles of primary and secondary air, and the products of incomplete versus complete combustion such as carbon monoxide and carbon dioxide. Key goals are achieving sufficient oxygen supply, high temperatures, and complete combustion to extract maximum heat from the fuel.