The document discusses body fluid compartments and their composition. It notes that:
- Total body water is about 42L for a 70kg male, divided into intracellular fluid (ICF) and extracellular fluid (ECF). ICF makes up about 28L while ECF is about 14L.
- ECF is further divided into interstitial fluid (ISF) and intravascular fluid (plasma). ISF is about 10L while intravascular fluid is 3.5L.
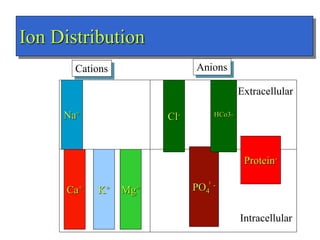
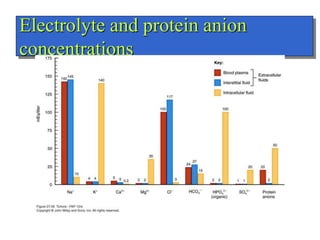
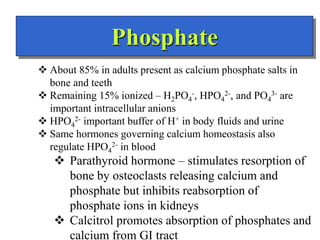
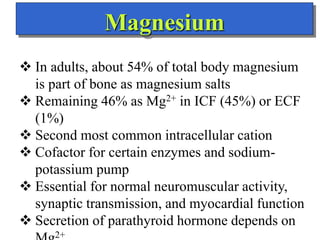
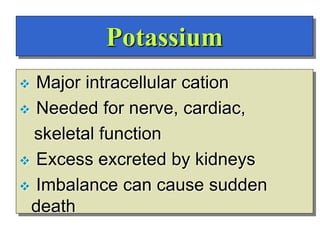
- The major ions in ICF are potassium and phosphates, while sodium and chloride predominate in ECF. Homeostasis of fluid volumes and ion concentrations is maintained by various mechanisms.
-














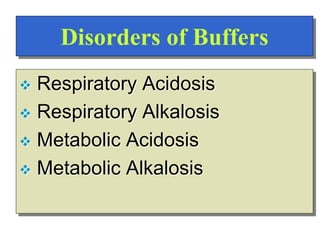
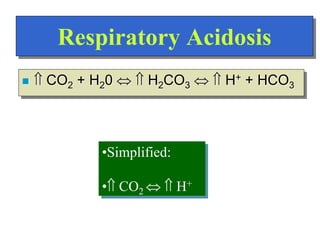
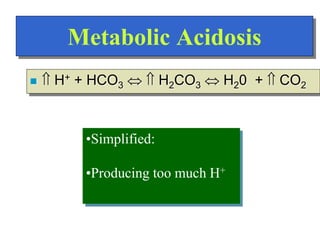
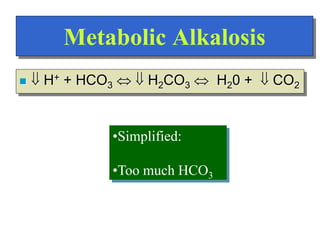
![Regulation of Acid-Base
Regulation of [H+]
Dependent upon:
Respiration
Kidneys
Chemical Buffers
Precise regulation necessary for peak
enzyme activity](https://image.slidesharecdn.com/bodyfluidcompartment-230203032236-7aec0620/85/Body-fluid-compartment-ppt-22-320.jpg)


![pH is logarithmic
pH = log 1/[H+]
= - log [H+]
= - log 0.00000004 Eq/L
pH = 7.4
Think of pH as ‘power of [H+]](https://image.slidesharecdn.com/bodyfluidcompartment-230203032236-7aec0620/85/Body-fluid-compartment-ppt-25-320.jpg)