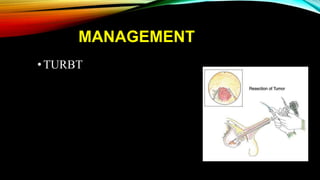
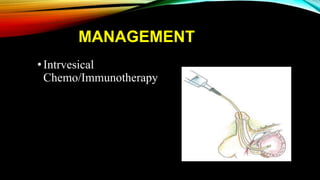
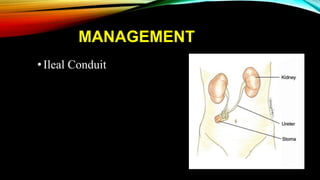
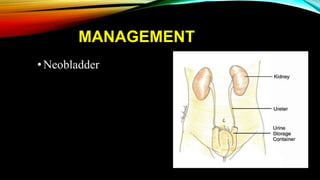
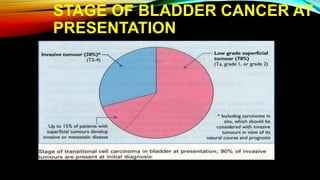
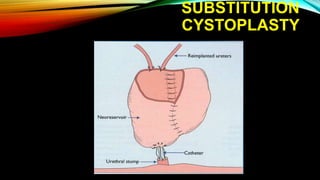
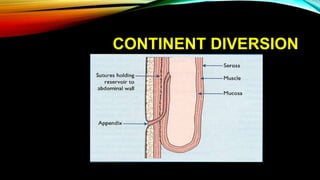
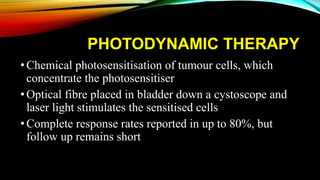
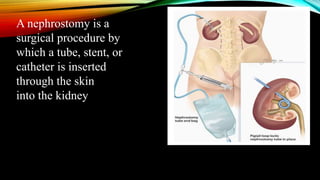
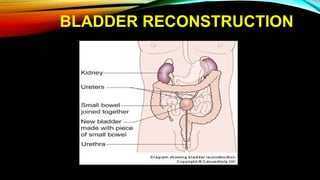
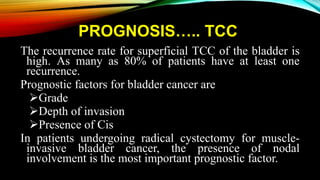
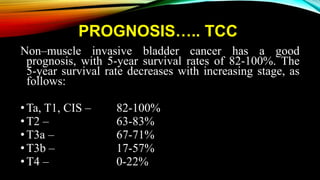
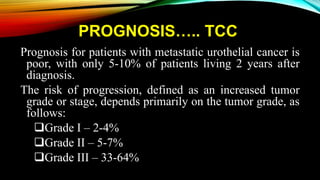
The document provides a comprehensive overview of bladder cancer management, including diagnosis, staging, and treatment options such as TURBT, intravesical therapies, and cystectomy. It details the prognostic factors and survival rates associated with various stages of bladder cancer, highlighting the importance of early intervention and monitoring due to the high recurrence rates. Additionally, the document discusses the implications of molecular genetics in bladder cancer progression and the advancements in surgical techniques for bladder reconstruction.



























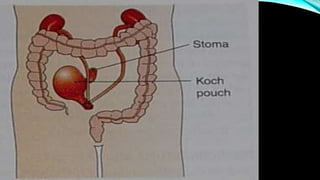
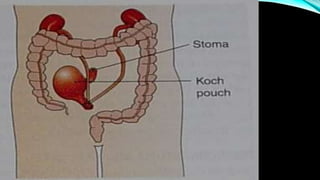

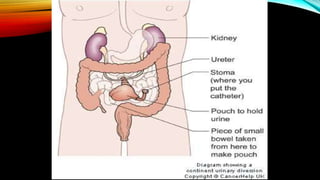
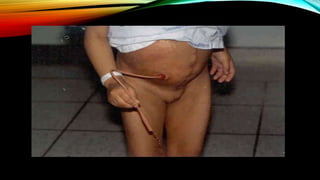

![PROGNOSIS….. SQUAMOUS
CELL CA
•Tumor stage, lymph node involvement, and tumor
grade have been shown to be of independent
prognostic value in SCC.[54, 55] However,
pathologic stage is the most important prognostic
factor.
•In SCC, the survival rate appears to be better with
radical surgery than with radiation therapy and/or
chemotherapy. In locally advanced tumors, however,
neoadjuvant radiation improves the outcome.](https://image.slidesharecdn.com/bladdermass-management-171201061122/85/Bladder-cancer-management-55-320.jpg)




