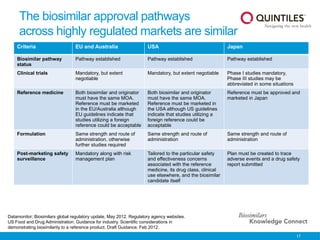
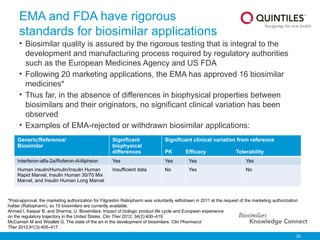
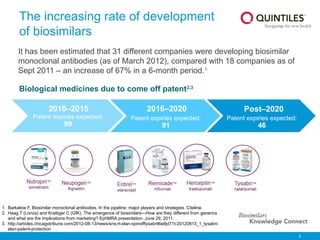
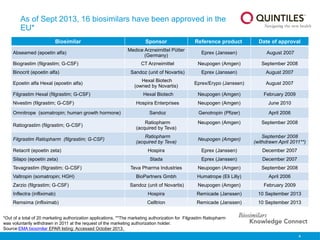
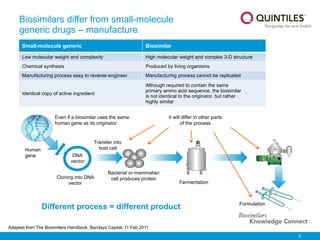
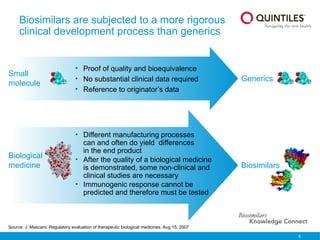
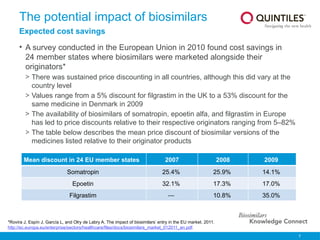
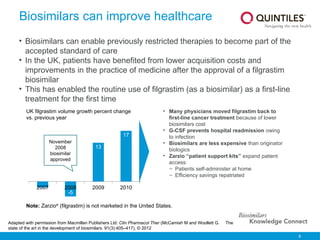
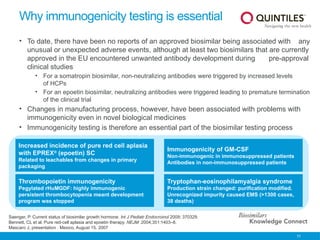
The document provides an overview of biosimilars, highlighting their development, regulatory context, and the cost-saving potential they offer in healthcare. It emphasizes that biosimilars, although highly similar to their originator biological medicines, undergo rigorous testing to ensure quality and safety, distinguishing them from small-molecule generics. The content also notes the growing interest in biosimilars due to patent expirations of biological medicines and their role in improving patient access to therapies.





![13
http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000408.jsp&mid=WC0b01ac058002958c
The BPCI Act appears in Title VII, subtitle A of the Patient Protection and Affordable Care Act, March 2010.
US Food and Drug Administration. Guidance for industry. Scientific considerations in demonstrating biosimilarity to a reference
product. Draft Guidance. Feb 2012.
Draft revisions
to Overarching
Guideline;
Quality
Guideline;
Non-clinical
and Clinical
Guideline
2004 2009 2011
2006 2007 2008 2012
2010 2013
2005
EMEA Legislative
Pathway
EMEA Regulatory
Guidance [Overarching
Guideline] under
revision
Product Class
Specific Guidelines:
Low molecular
weight heparin,
recombinant
Interferon-alpha
Product Class
Monoclonal
antibodies –
non-clinical and
clinical issues
Product Class
Specific Guideline:
Erythropoietin
(revised)
Quality Guideline;
Non-Clinical and
Clinical Guideline
(under revision )
Product Class
Specific Guidelines:
Insulin, G-CSF,
Somatropin
Product Class
Immunogenicity
assessment of
monoclonal
antibodies
Public Health
Service Act
amended to
allow the
approval of
biosimilars
Overarching Draft
Guidelines on
biosimilars
Europe US
Biosimilar regulations in EU and
USA: different stages of development
• The EU pioneered the development of
biosimilar regulations
• US overarching guidelines issued
Draft revisions to
Product Class
Specific Guideline:
Insulin, low molecular
weight heparin
Product
Class
Follicle
stimulating
hormone,
Interferon-
beta](https://image.slidesharecdn.com/biosimilarsknowledgeconnectslideresource-241230105948-891d68b4/85/Biosimilars-Knowledge-Connect-slide-resource-pptx-13-320.jpg)