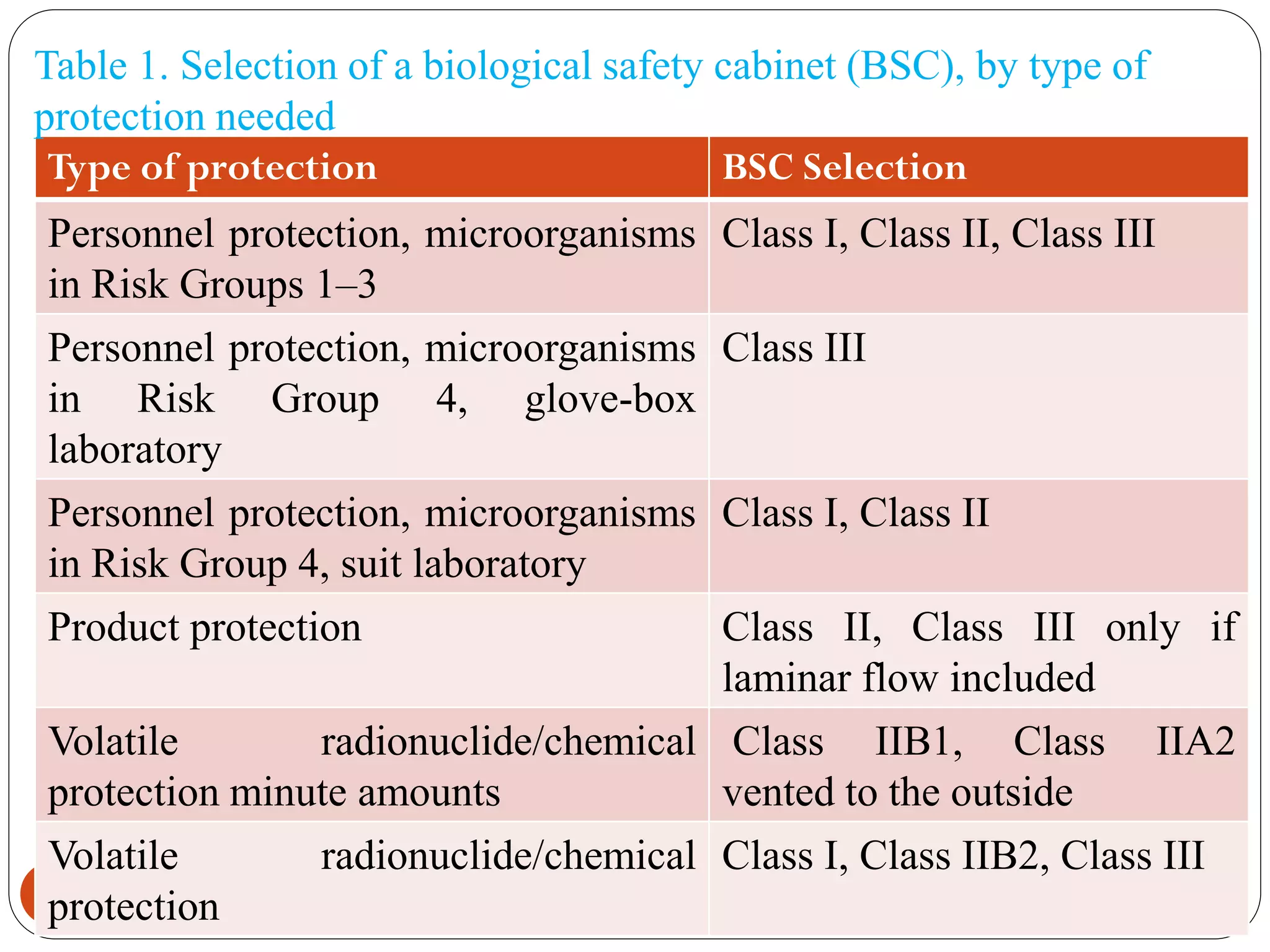
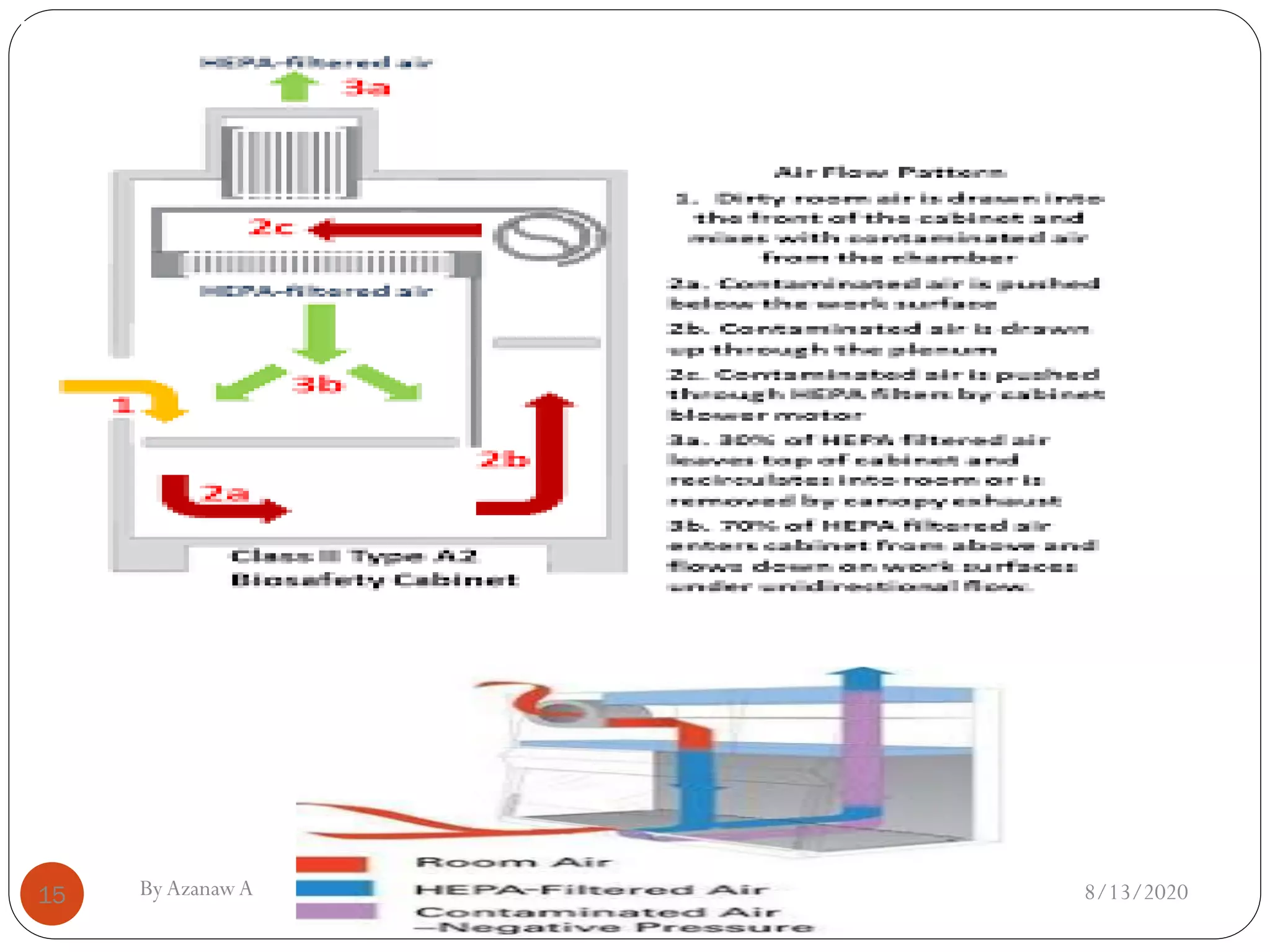
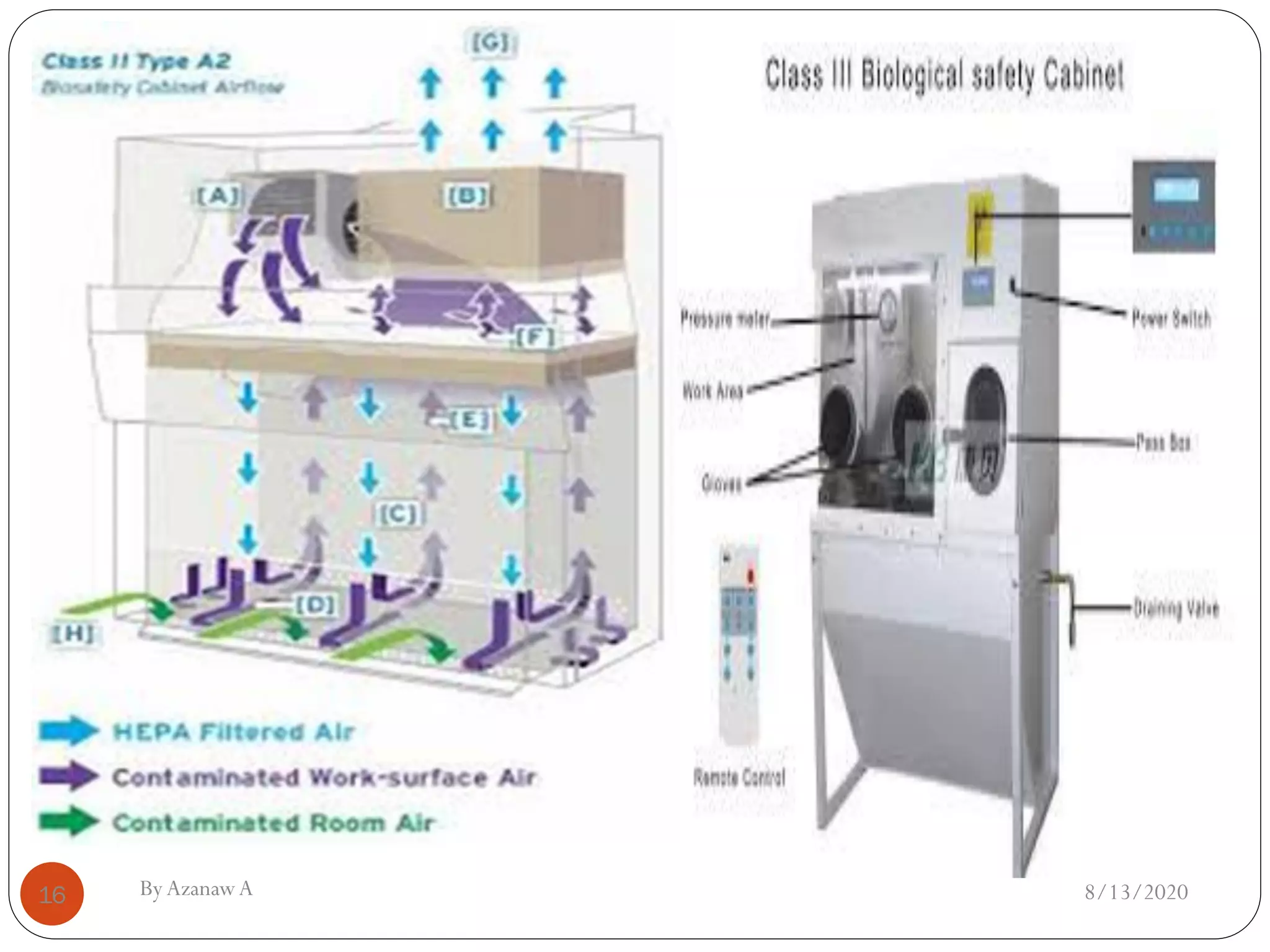
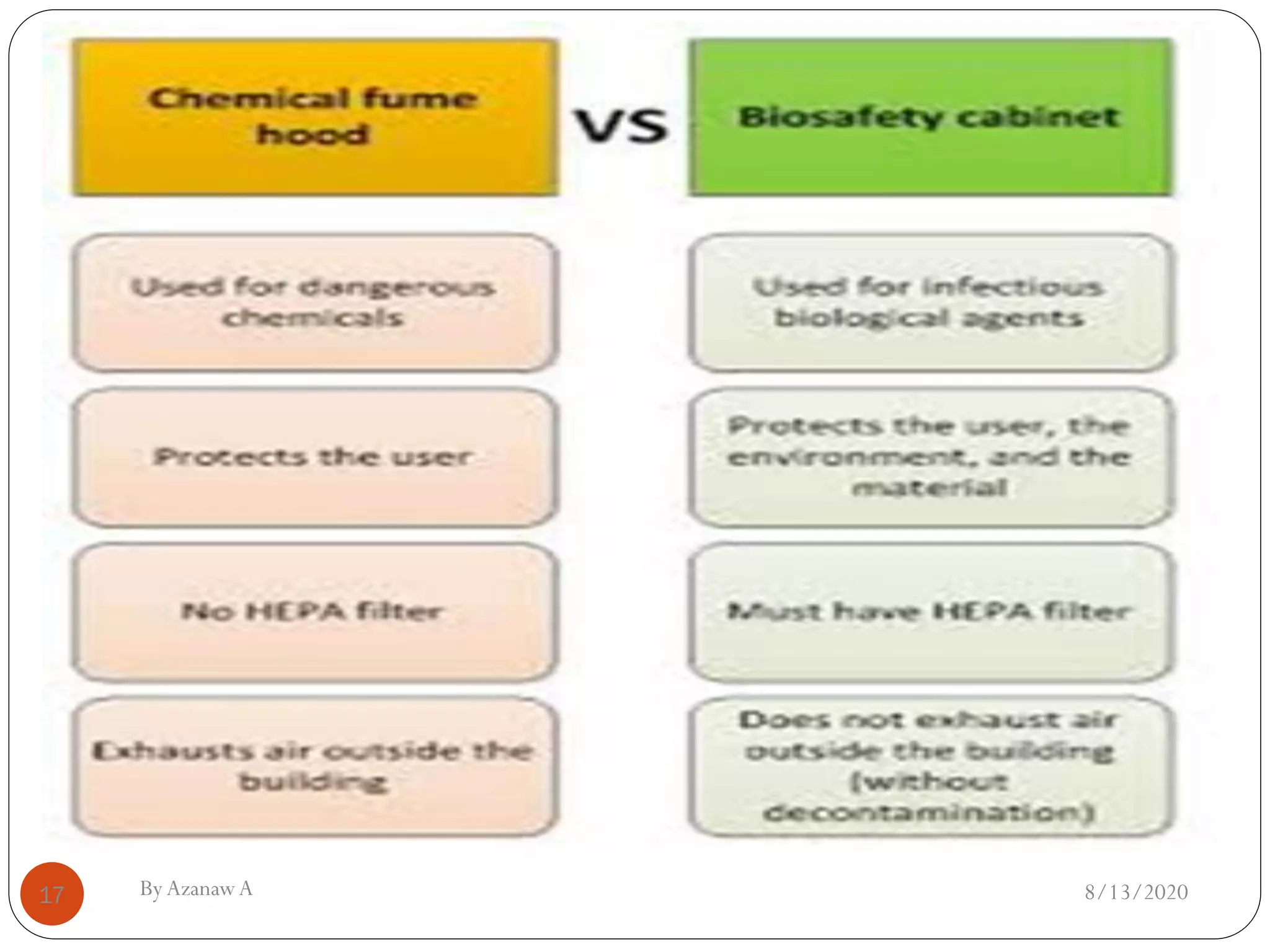
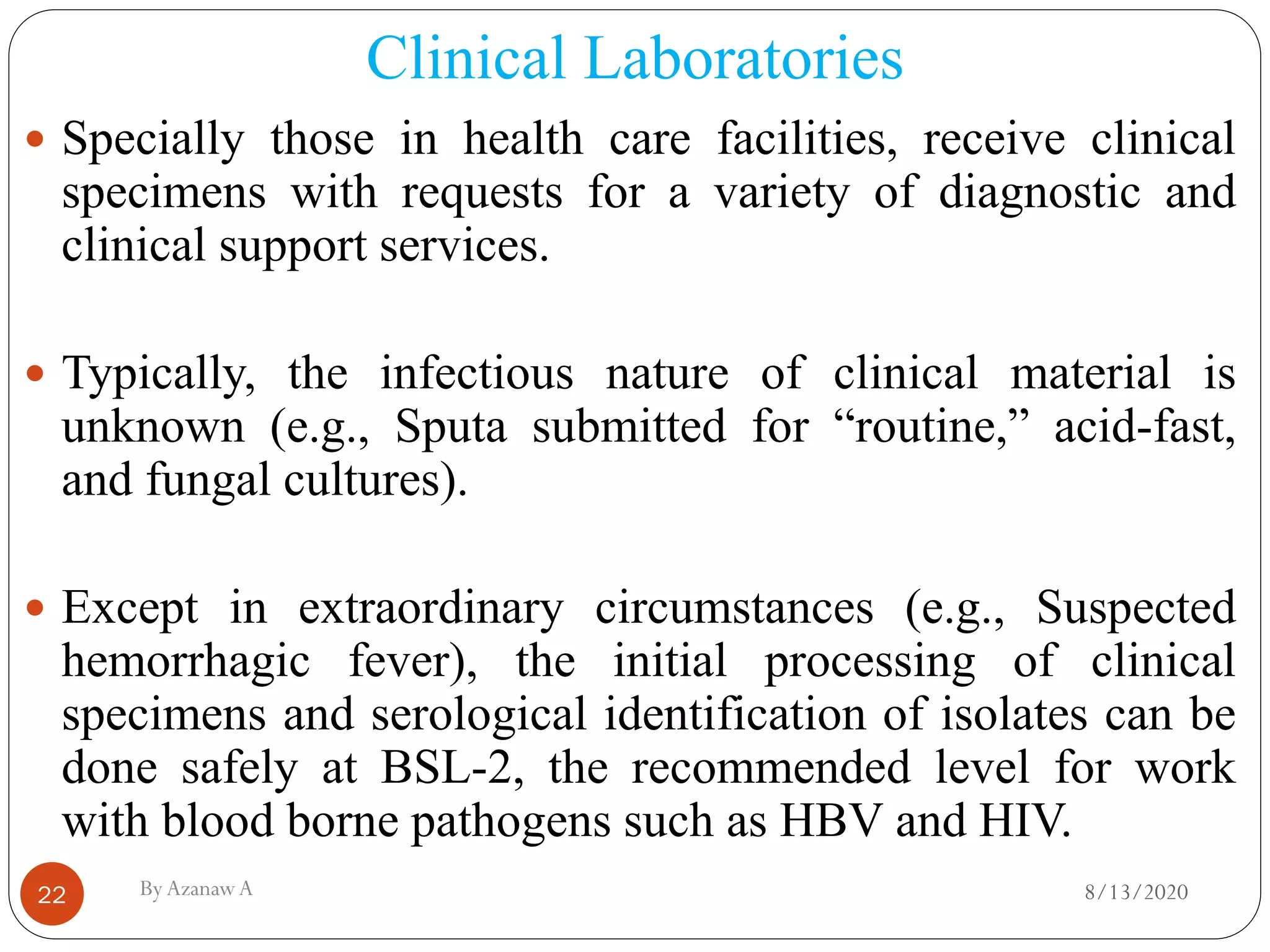
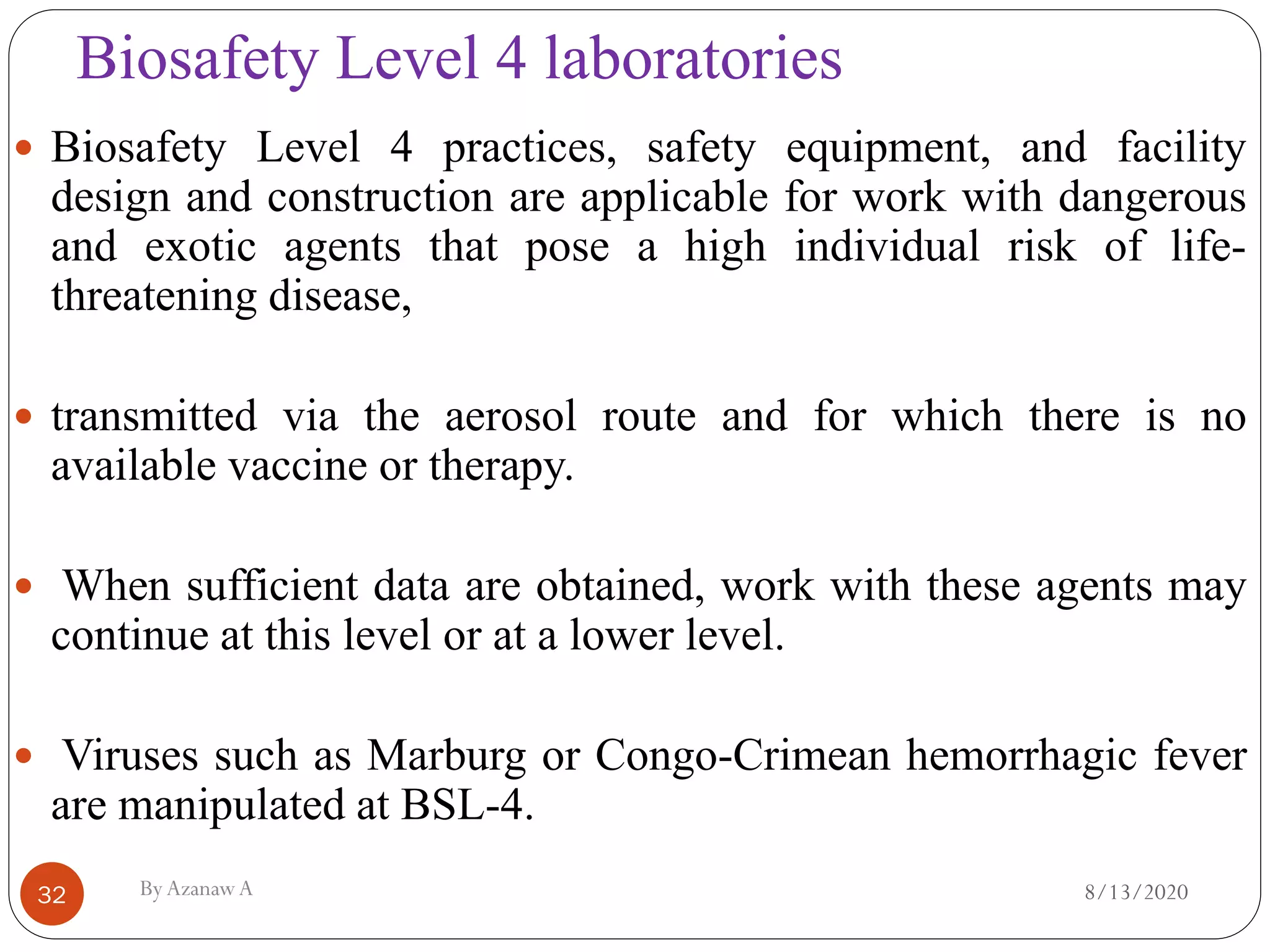
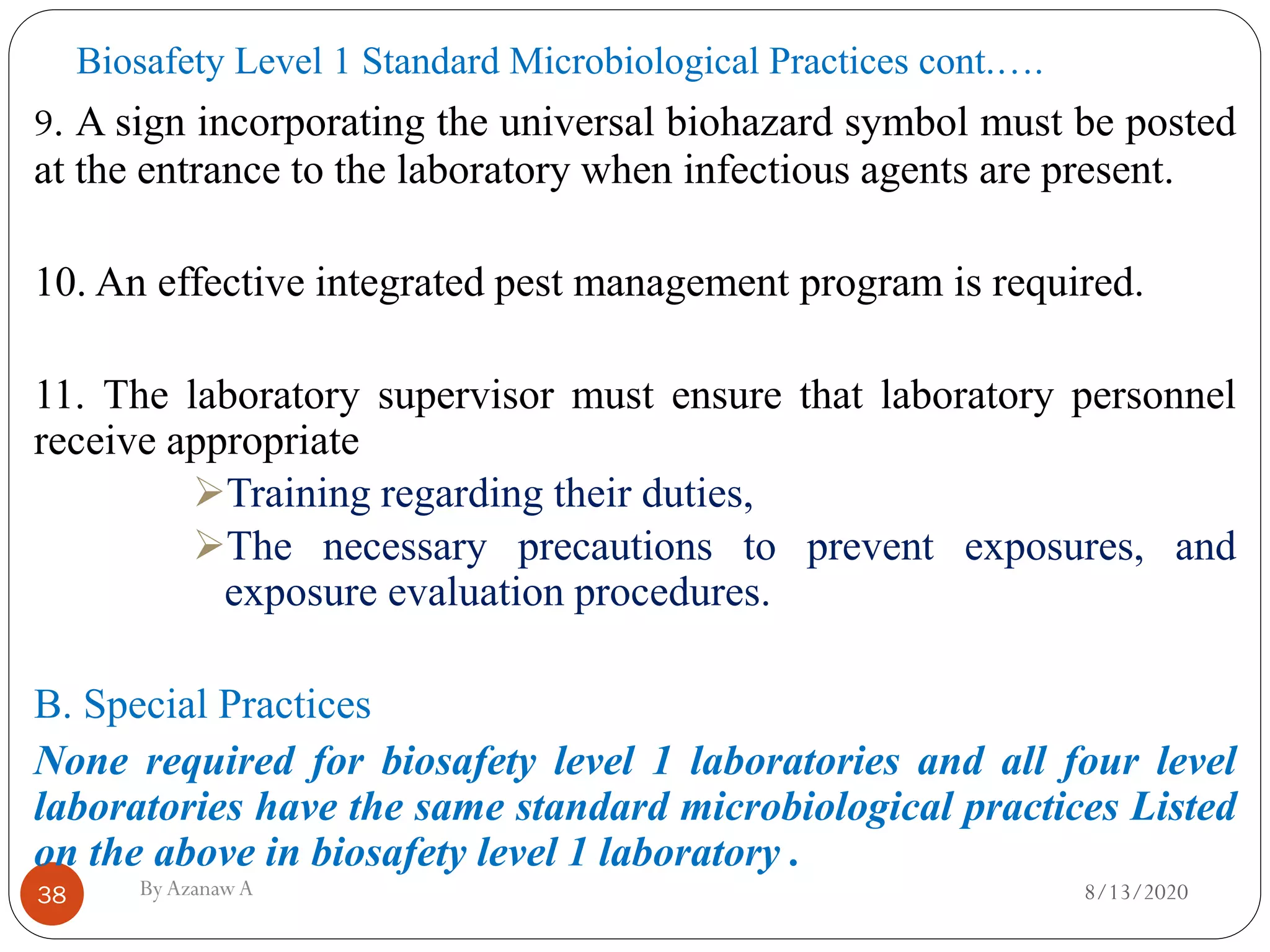
This document outlines biosafety practices in microbiology laboratories. It discusses the principles of biosafety including containment, personal protective equipment (PPE), and facility design. The three classes of biological safety cabinets are described, which provide different levels of protection depending on the agent handled. Secondary barriers like specialized ventilation and controlled access zones are important facility design considerations. Biosafety levels are assigned based on the risk group of the agent. Guidelines also cover animal facilities, clinical laboratories, shipping infectious materials, and regulations around select agents. The goal is to protect laboratory workers and the outside environment from exposure to hazardous biological materials.