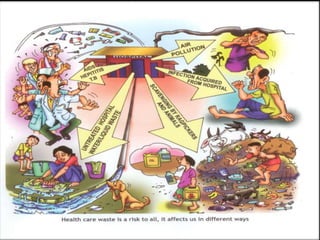
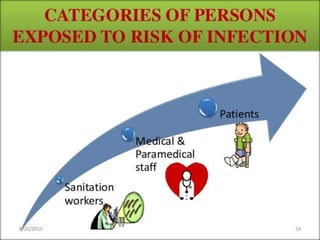
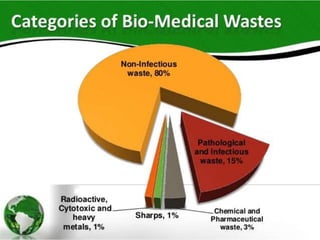
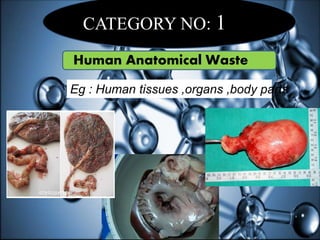
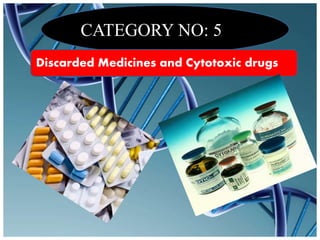
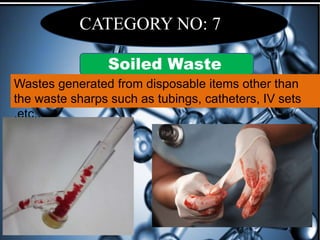
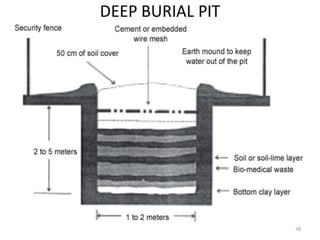
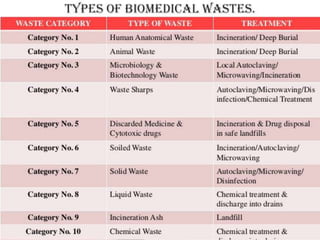
The document defines biomedical waste and provides classifications. It notes major sources are healthcare facilities while minor sources include clinics and funeral homes. Biomedical waste is classified as non-hazardous or hazardous, with infectious waste making up 15-18% of hazardous waste. The document details regulations for handling, storing, transporting, and disposing of different categories of biomedical waste, including through incineration, autoclaving, deep burial or landfilling. Proper training and coordination with outside agencies is emphasized.




![Classification of Bio‐Medical Waste
Biomedical waste
Non-Hazardous
[75-90%]
Hazardous
[10-25%]
Infectious [15-18%] Other Hazardous [5-7%]
Non-sharps
Sharps
Plastic disposables
Liquid wastes
Radioactive waste
Discarded glass
Pressurized containers
Chemical waste
Cytotoxic waste
Incinerator ash](https://image.slidesharecdn.com/jabezbiomedicalwaste-171119164556/85/biomedical-waste-5-320.jpg)