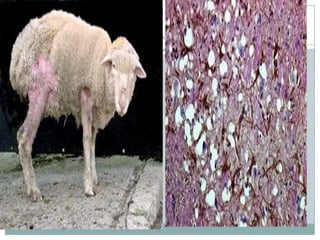
Protein is composed of amino acids and is necessary for proper growth and function of the human body. It has many important biological functions including structural, enzymatic, transport, motile, regulatory, and storage functions. There are different types and levels of protein structure. Changes in protein shape can cause diseases like prion diseases and Creutzfeldt-Jakob disease which affect the nervous system. These diseases are difficult to diagnose and are generally fatal.