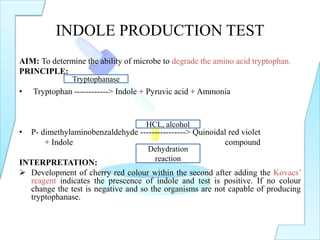
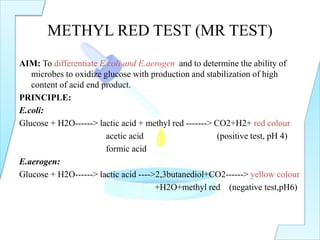
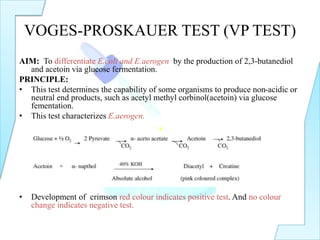
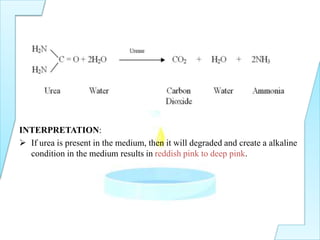
The document outlines various biochemical tests in microbiology used to determine microbial capabilities, including the degradation of tryptophan, glucose fermentation, urease activity, and citrate utilization. Key tests such as the indole production test, methyl red test, Voges-Proskauer test, and citrate utilization test are explained, including their principles, interpretations, and indicators for positive and negative results. The references cited support the methodologies described in the tests.