Water is essential for life and makes up a large percentage of the human body. The document discusses several key points about water and acid-base balance in the human body:
1) Water is involved in many critical functions like acting as a solvent, participating in metabolic reactions, and regulating body temperature. The body precisely controls water balance through mechanisms like thirst.
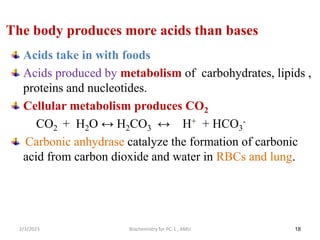
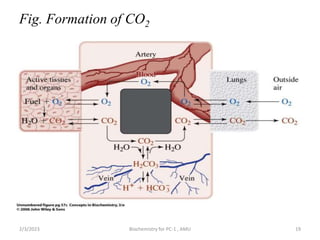
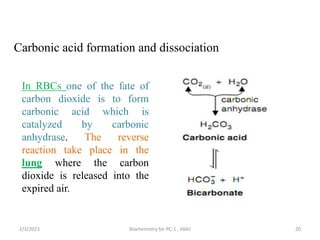
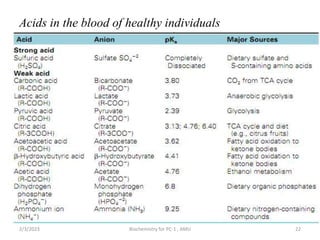
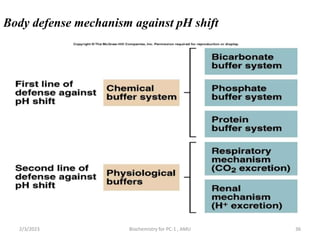
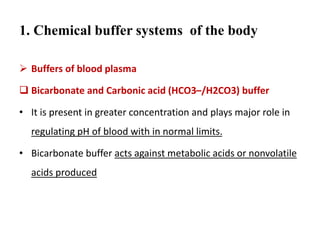
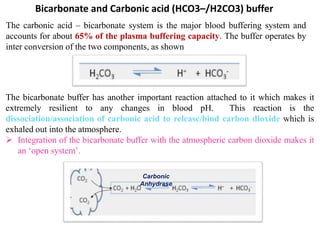
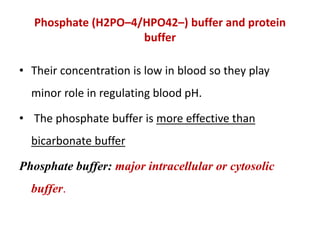
2) The body produces acids through cellular metabolism which can lower pH. However, several buffer systems precisely regulate pH, including bicarbonate buffers, phosphate buffers, and protein buffers.
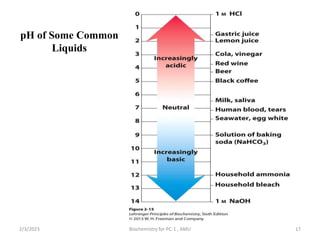
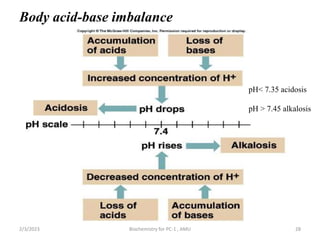
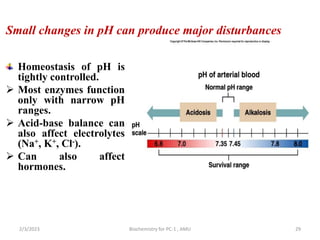
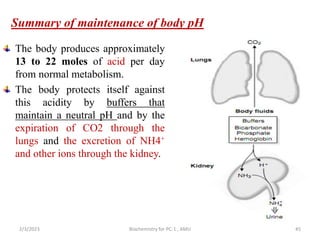
3) When the pH shifts outside the narrow range of 7.35-7.45, it can cause acidosis or alkalosis respectively. The lungs,












![Acids, bases and pH
Acids are compounds that donate a hydrogen ion to a
solution and that lowers the pH
Bases are compounds that accept hydrogen ions and that
raises the pH.
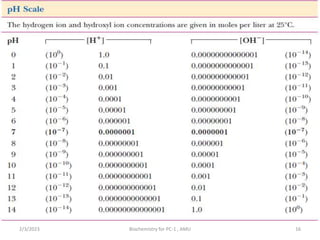
pH is the negative logarithm of hydrogen ion
concentration [H+].
i.e. pH= - log [H+]
The most important factor ,which must be considered in the
regulation of acid base balance is the hydrogen ion
concentration.
2/3/2023 Biochemistry for PC-1 , AMU 15](https://image.slidesharecdn.com/biochem2-230203170256-c3a9cf9b/85/Biochem2-pptx-15-320.jpg)






![Protein Buffer Systems
The intracellular as well as extracellular proteins can also contribute to the buffering
capacity of body fluids.
The proteins carry ionisable side chains on their amino acids which can give rise
to the protein anions (Pr-) and free protons. Similarly, the protein anions can absorb
H+ ions to take care of excess acidity.
Proteins act as base (Pr-) under acidic conditions and absorb the H+ ions released by
the acids; and Act as acids (Pr-H) under alkaline conditions releasing the protons to
neutralize the alkali.
Histidine is the most effective amino acid with a pKa of 6.0.
Albumin has 16 histidine residues which help it to act as an effective buffer in
plasma.
Protein buffer contributes about 4% of the plasma buffering capacity.
Pr-H Pr- + [H+]](https://image.slidesharecdn.com/biochem2-230203170256-c3a9cf9b/85/Biochem2-pptx-41-320.jpg)






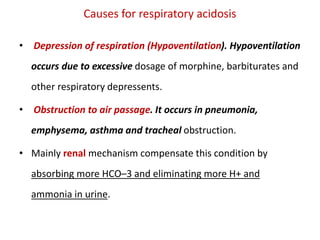
![ Respiratory acidosis.
• It is due to more plasma PCO2 level.
Increased [CO2] that leads to increased [H2CO3].
Caused by increased retention of CO2 in the lung or
inhalation of ↑[CO2
Acidosis ( acidemia): pH < 7.35](https://image.slidesharecdn.com/biochem2-230203170256-c3a9cf9b/85/Biochem2-pptx-54-320.jpg)