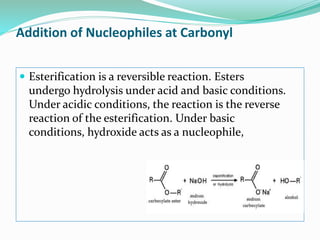
1. The document discusses various reactions involving esters such as esterification, hydrolysis, addition of nucleophiles, reduction, and uses as protecting groups.
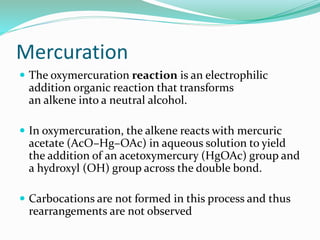
2. It also covers bromination and mercuration reactions on alkenes, noting that bromination proceeds with anti-stereochemistry and is stereospecific, while mercuration forms alcohols in three steps without rearrangements.
3. Additional topics include brominated vegetable oil, uses of esters in nature, and reactions of esters like Claisen condensation.