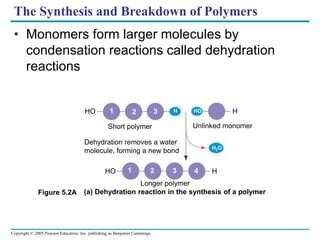
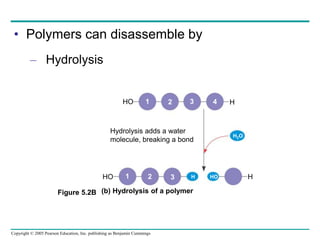
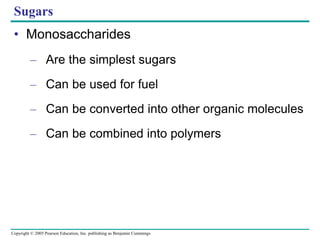
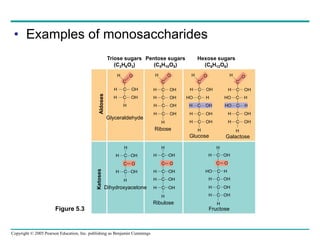
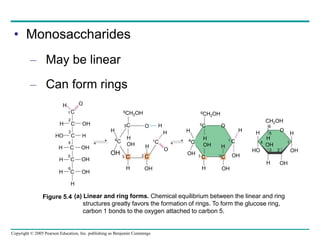
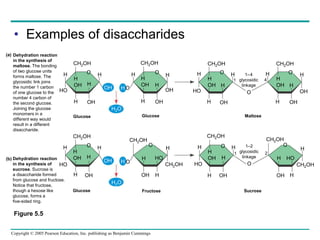
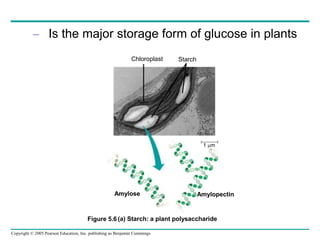
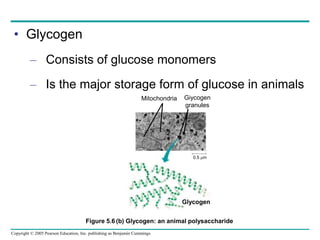
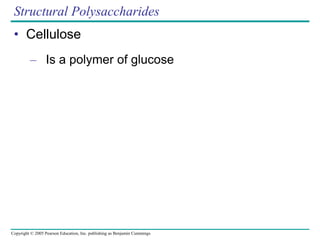
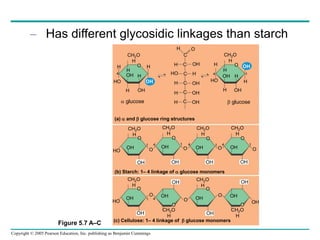
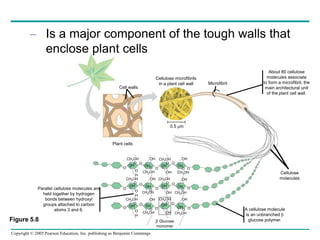
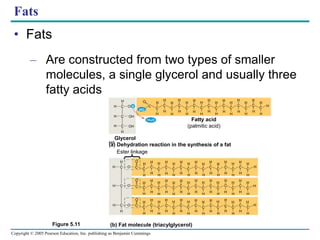
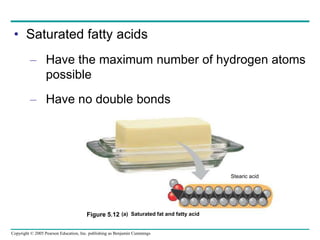
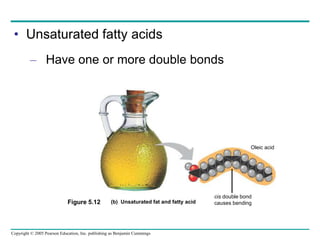
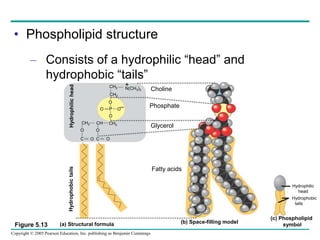
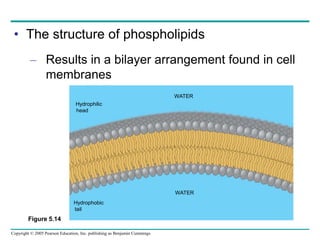
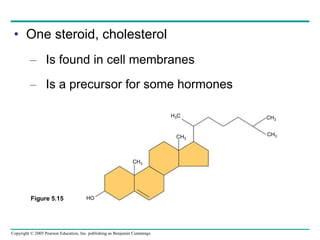
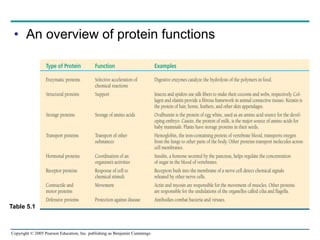
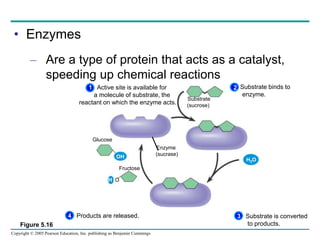
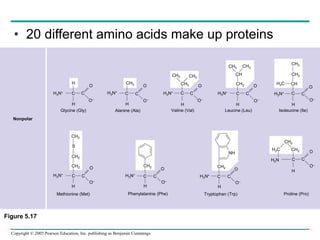
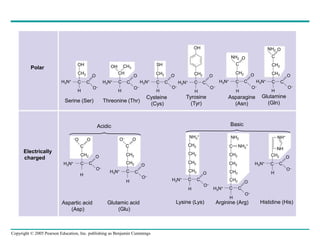
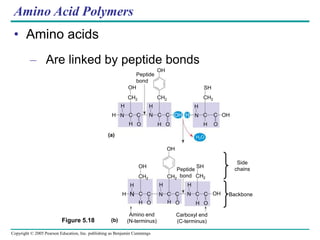
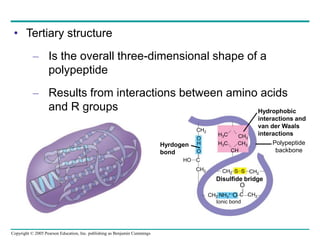
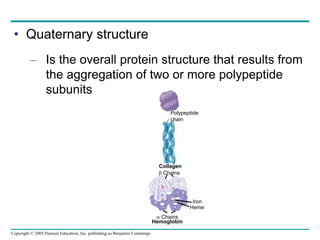
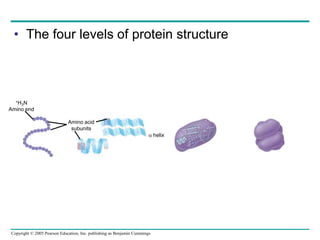
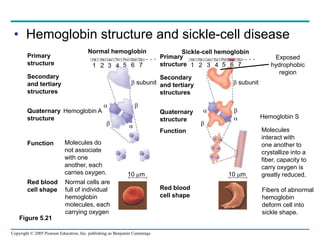
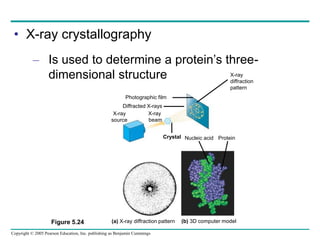
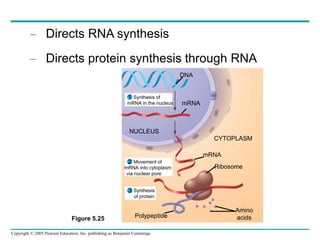
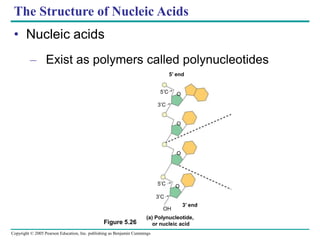
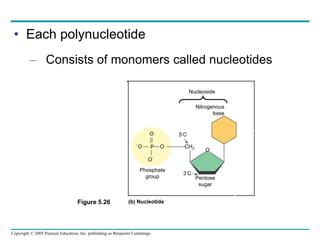
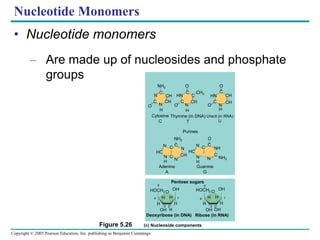
The document discusses the structure and function of macromolecules, emphasizing that they are large polymers formed from smaller monomers. It covers the synthesis and breakdown of polymers, the diversity of biological polymers like carbohydrates, proteins, and lipids, and details the roles of polysaccharides, fats, and proteins in organisms. Key points include the formation processes of monomers into polymers and the unique functions and structures of each macromolecule class.