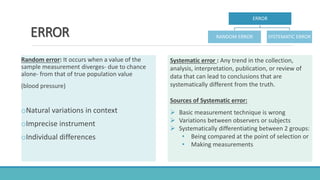
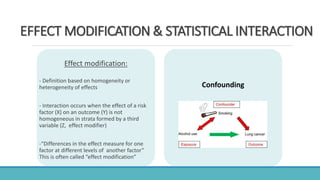
Here is an alphabetical list of biases mentioned in the document along with their type and which study design they can occur in:
- Attrition bias (selection bias) - Can occur in cohort studies
- Berksonian bias (selection bias) - Can occur in observational studies
- Information bias/Measurement bias - Can occur in all study designs
- Interview bias (information bias) - Can occur in case-control and cohort studies
- Lead time bias (selection bias) - Can occur in screening studies
- Length time bias (selection bias) - Can occur in screening studies
- Non-differential misclassification bias (information bias) - Can occur in all study designs
- Overdiagnosis bias (selection bias