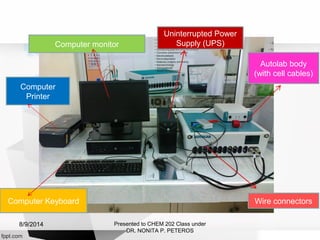
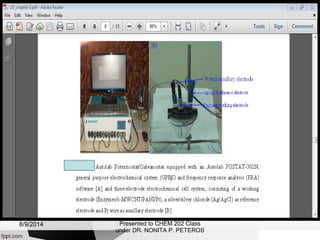
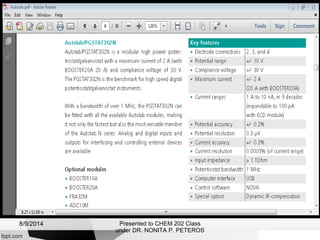
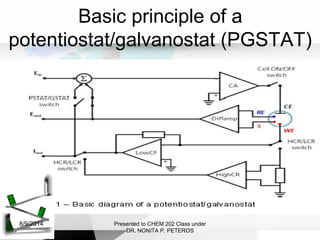
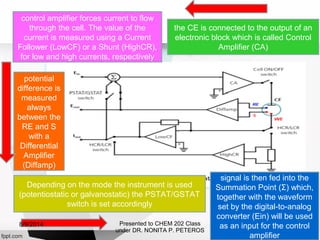
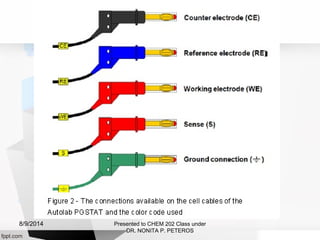
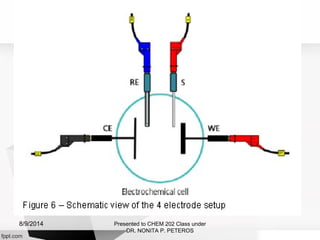
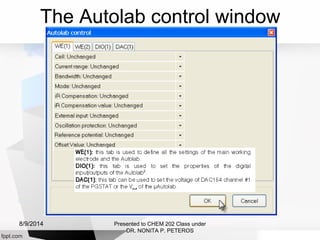
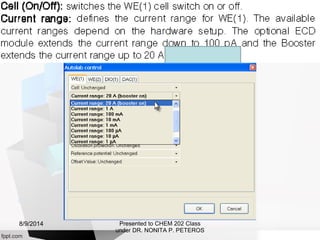
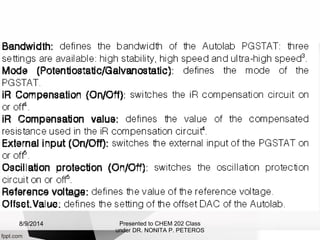
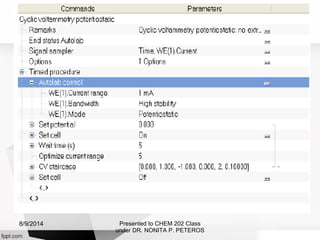
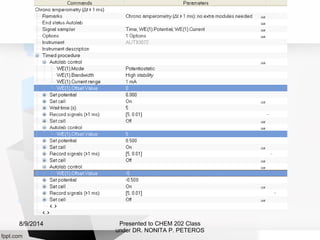
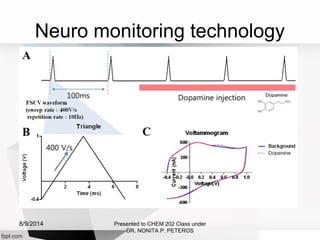
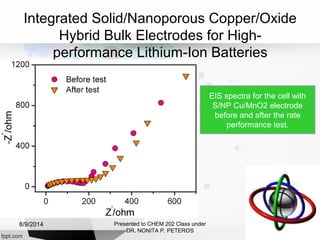
Autolab is an electrochemical measurement system consisting of a potentiostat/galvanostat and data acquisition system. It can be used to perform techniques like cyclic voltammetry and chronoamperometry. The system controls the potential or current applied to a three-electrode cell containing a working, counter, and reference electrode. Users prepare an experiment procedure specifying parameters like potential ranges and scan rates, then view and analyze the resulting electrochemical data.