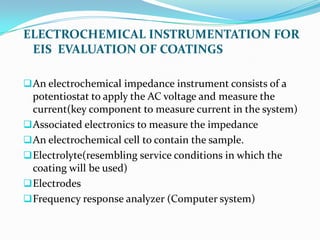
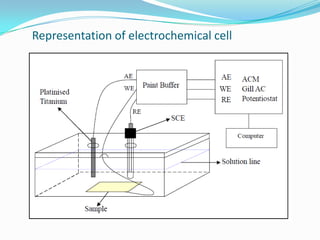
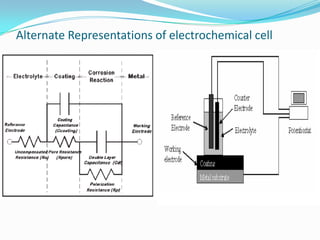
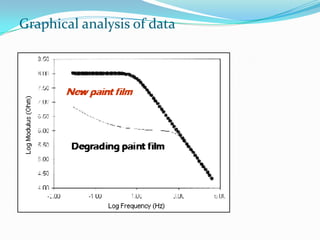
Electrochemical impedance spectroscopy (EIS) is a technique used to characterize coated metals and monitor the degradation of coatings over time. EIS involves applying an AC voltage to a coated metal sample immersed in an electrolyte and measuring the impedance over a range of frequencies. Changes in impedance parameters such as coating capacitance, pore resistance, and polarization resistance can provide information about the coating's condition and failure processes. EIS is able to detect coating deterioration earlier and more sensitively than traditional tests like salt spray testing. It generates quantitative data to evaluate coatings and optimize formulations while reducing testing time compared to other techniques.