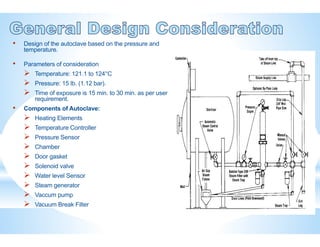
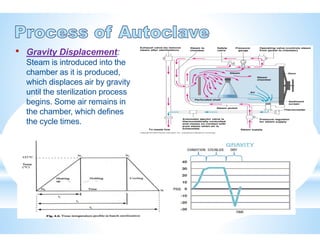
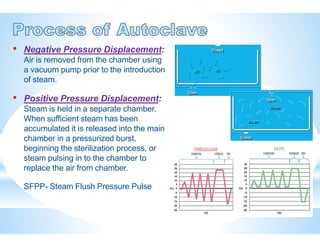
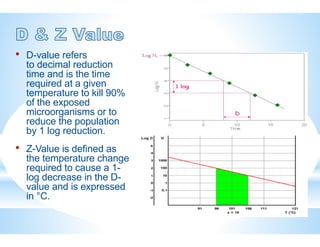
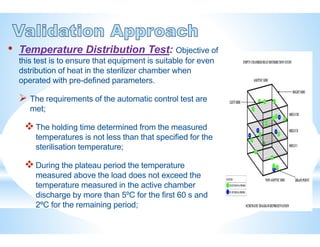
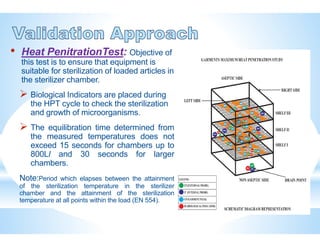
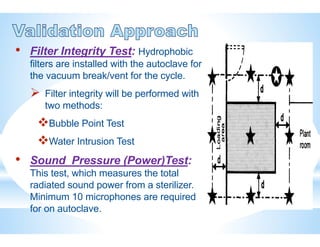
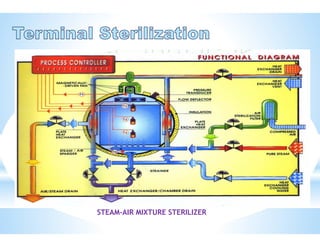
The document provides an overview of autoclaves, including their history, operational principles, and various types used for sterilization. It highlights key specifications, processes, and testing methods required to ensure effective sterilization and safety in multiple industries. Additionally, it discusses regulatory standards and qualifications necessary for autoclave performance validation.