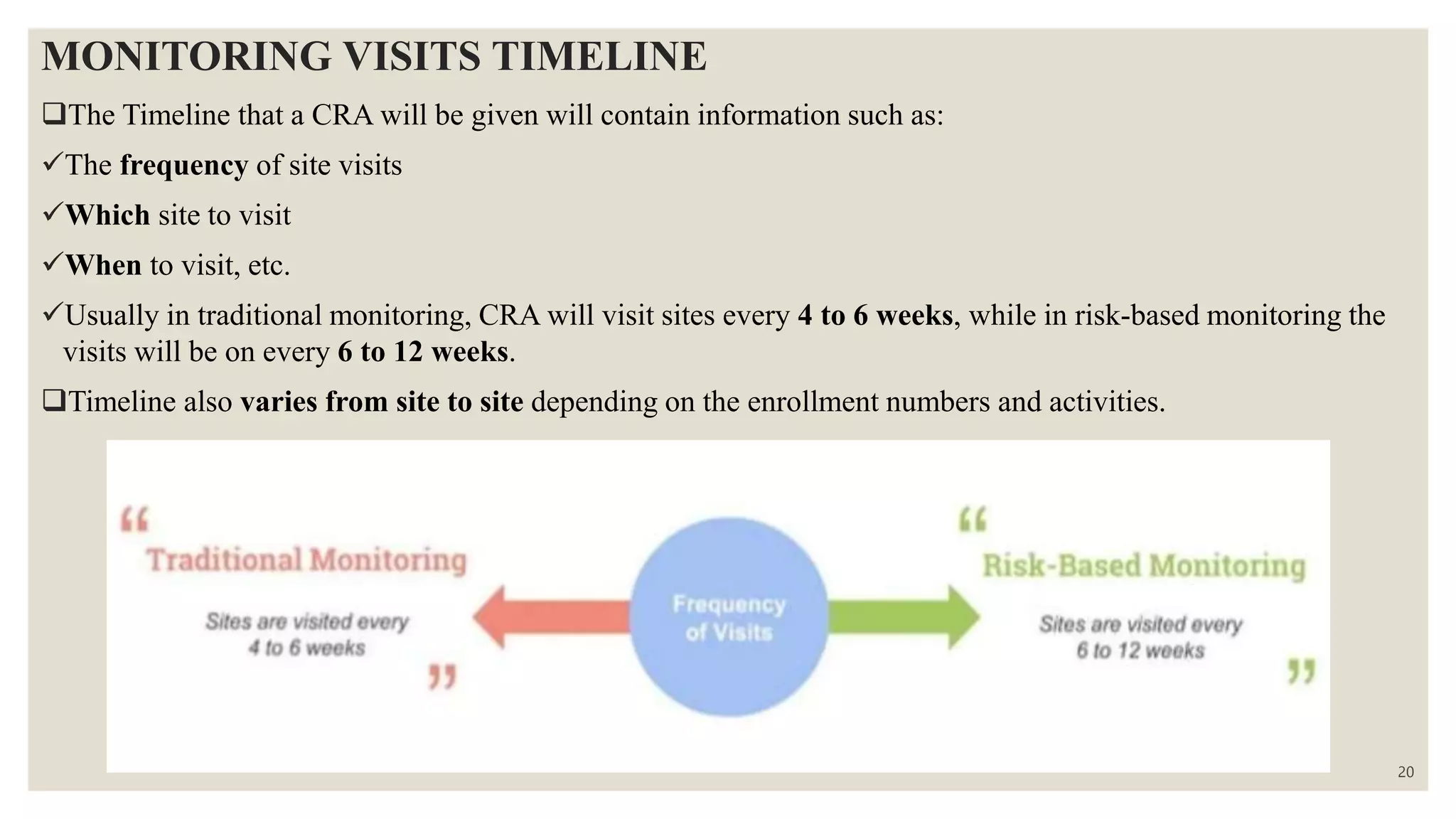
The document outlines the role and responsibilities of monitors in clinical trials, emphasizing the importance of investigational product accountability and adherence to protocols. It details the monitoring process, types of monitoring visits (site selection, initiation, routine, and close-out), and the qualifications required for clinical research associates (CRAs). Additionally, it describes the procedures for ensuring compliance and conducting thorough monitoring throughout the study lifecycle.