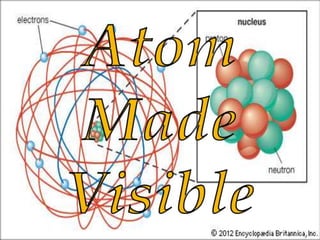
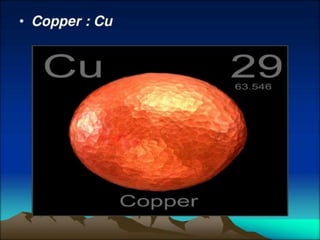
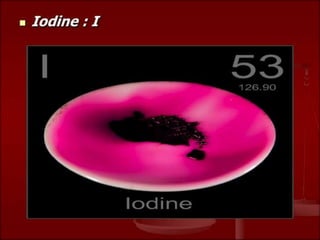
This document provides information about the structure and composition of atoms. It discusses the discovery of subatomic particles like electrons, protons and neutrons through the models of Thomson and Rutherford. It explains that atoms are very small, around 10-10 meters, and describes the shells and number of electrons in the K, L, M and N shells. Tables listing common elements and their symbols are included. The document also defines molecules as groups of atoms bonded together, and provides examples of monoatomic and diatomic molecules like hydrogen, oxygen and ozone. It introduces the concept of ions as charged particles in compounds.