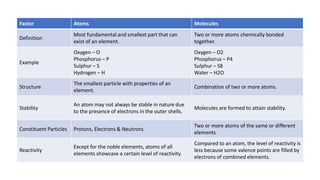
An atom is the smallest unit of matter that retains the properties of an element. It consists of a nucleus containing protons and neutrons, surrounded by electrons. A molecule is made up of two or more atoms bonded together. Molecules exhibit the properties of their constituent elements and can be broken down into atoms. Atoms are the fundamental building blocks that make up all matter in the universe and were formed after the Big Bang. While once thought to be indivisible, atoms can undergo chemical reactions that separate, combine, or rearrange them.