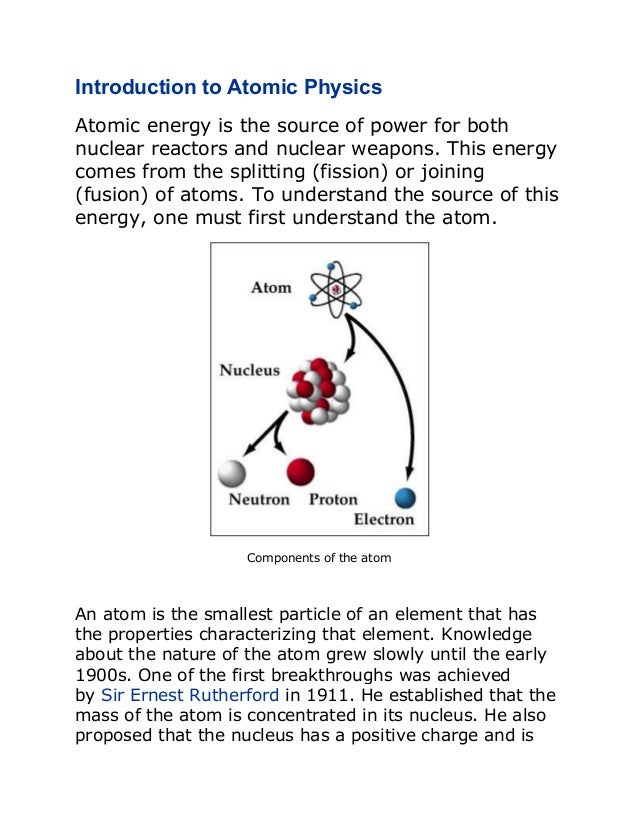
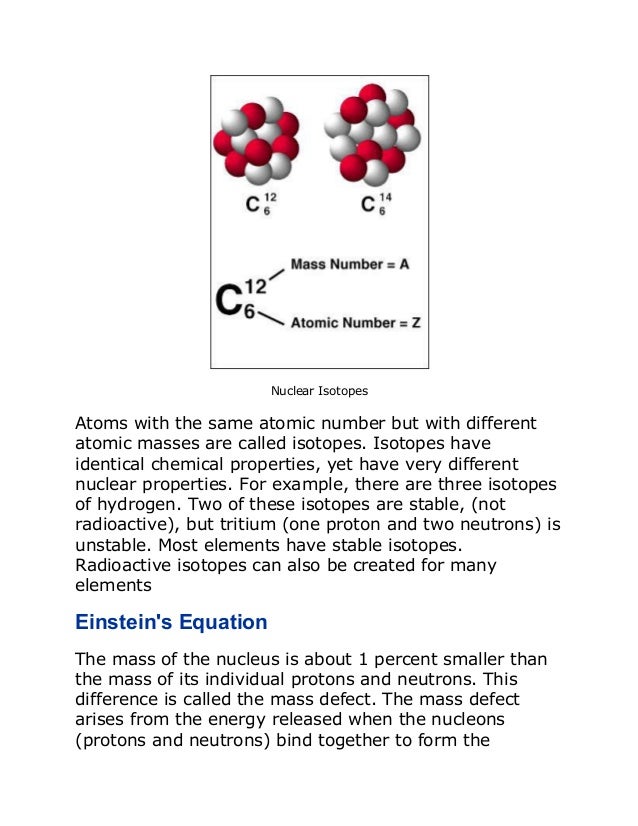
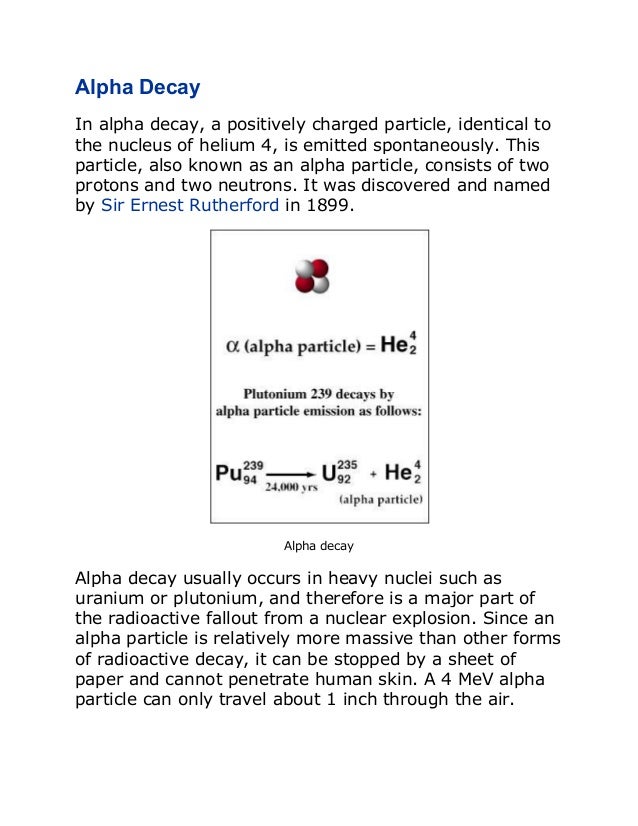
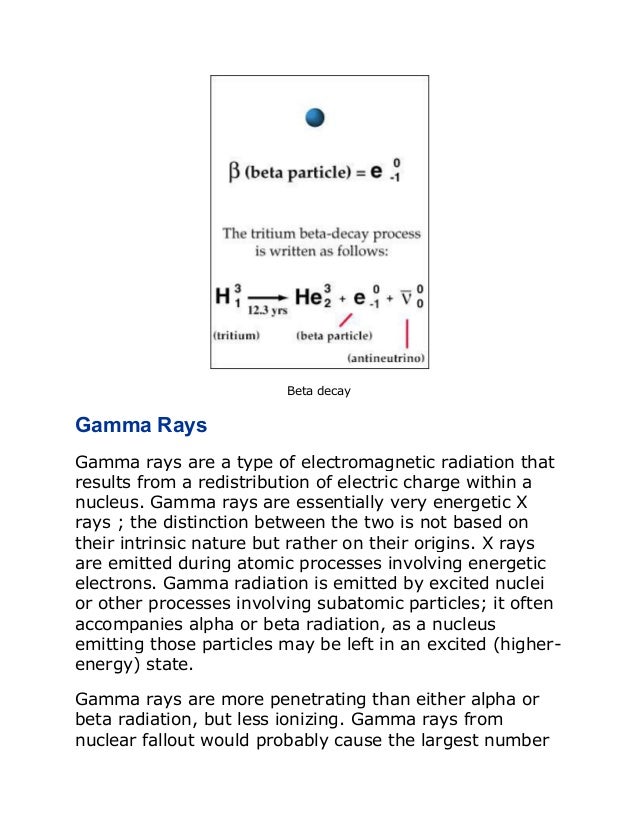
An atom consists of a small, dense nucleus surrounded by electrons. The nucleus contains protons and neutrons, and the number of protons determines the element. Atoms can undergo radioactive decay through processes like alpha decay (emitting helium nuclei), beta decay (emitting electrons or positrons), or gamma ray emission. Einstein's equation E=mc2 shows that a small amount of mass can be converted into a large amount of energy through nuclear reactions like fission and fusion.