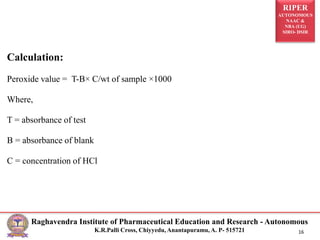
The document discusses the iodine value, rancidity, and peroxide value in the context of pharmaceutical analysis. It details methods for measuring the iodine value to assess unsaturation in oils, examines the types and causes of rancidity, and explains the significance of peroxide value as an indicator of oil degradation. The text is structured around analytical procedures, calculations, and harmful effects associated with rancid food.