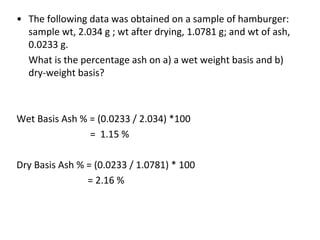
This document discusses methods for determining the ash and mineral content of foods. Ash content refers to the total amount of minerals, which is determined by heating to remove organic matter. Mineral content refers to specific inorganic components. There are three main types of ashing: dry ashing uses high heat to burn organic matter; wet ashing uses acids to digest organic matter; and plasma ashing uses ionized gases. The chosen method depends on the analysis and equipment. Sample preparation is also important to obtain representative and contaminant-free results.