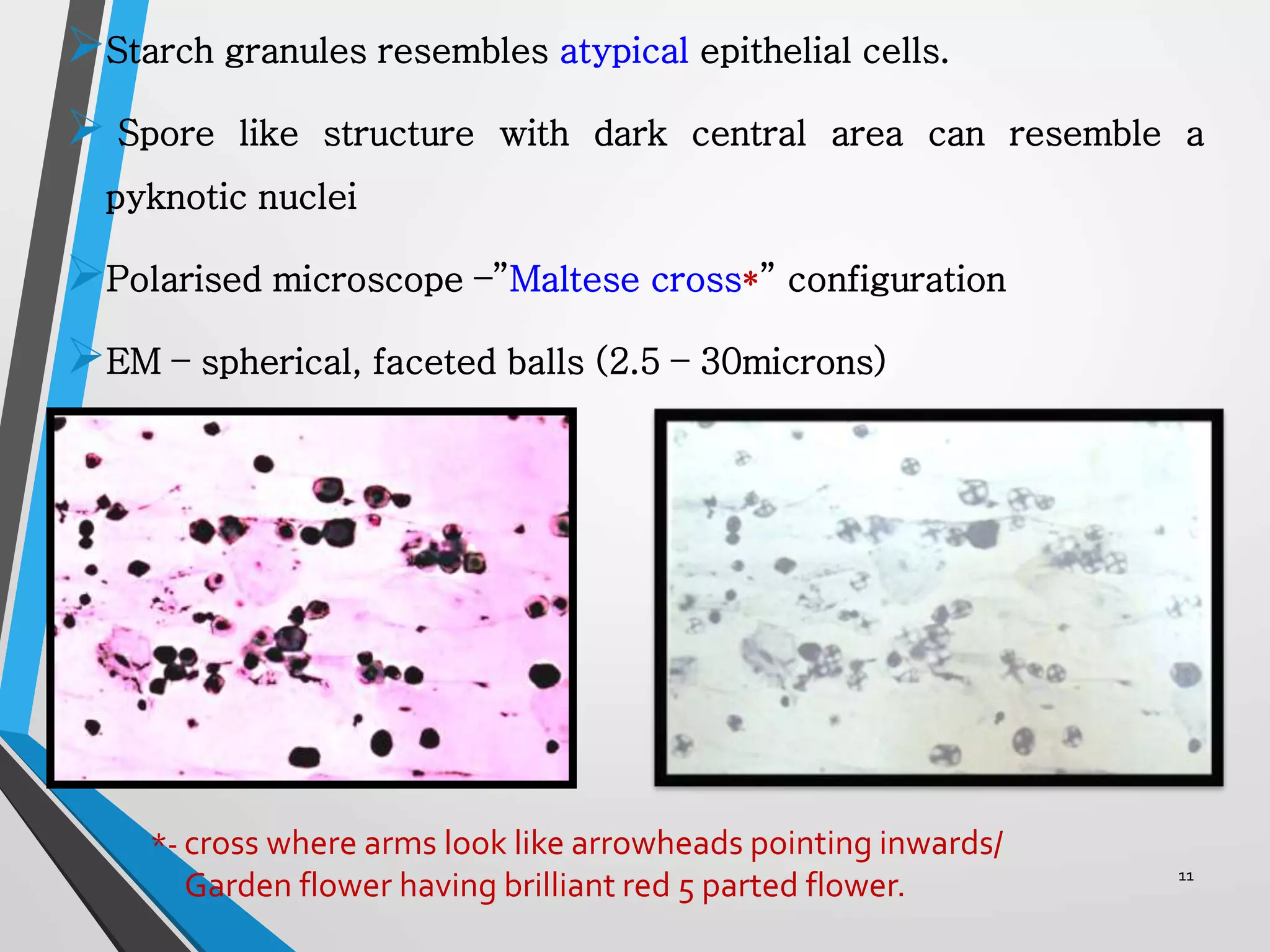
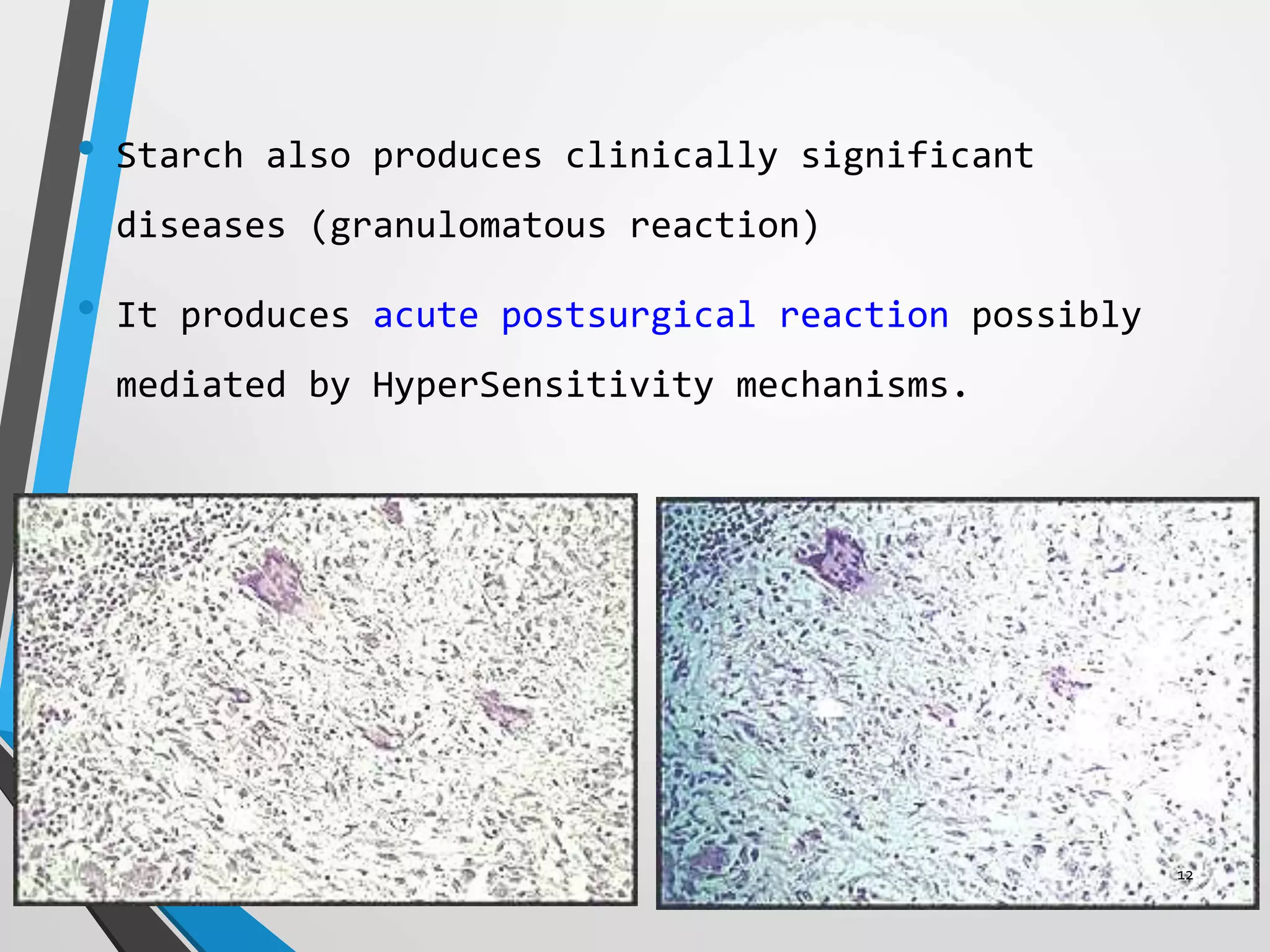
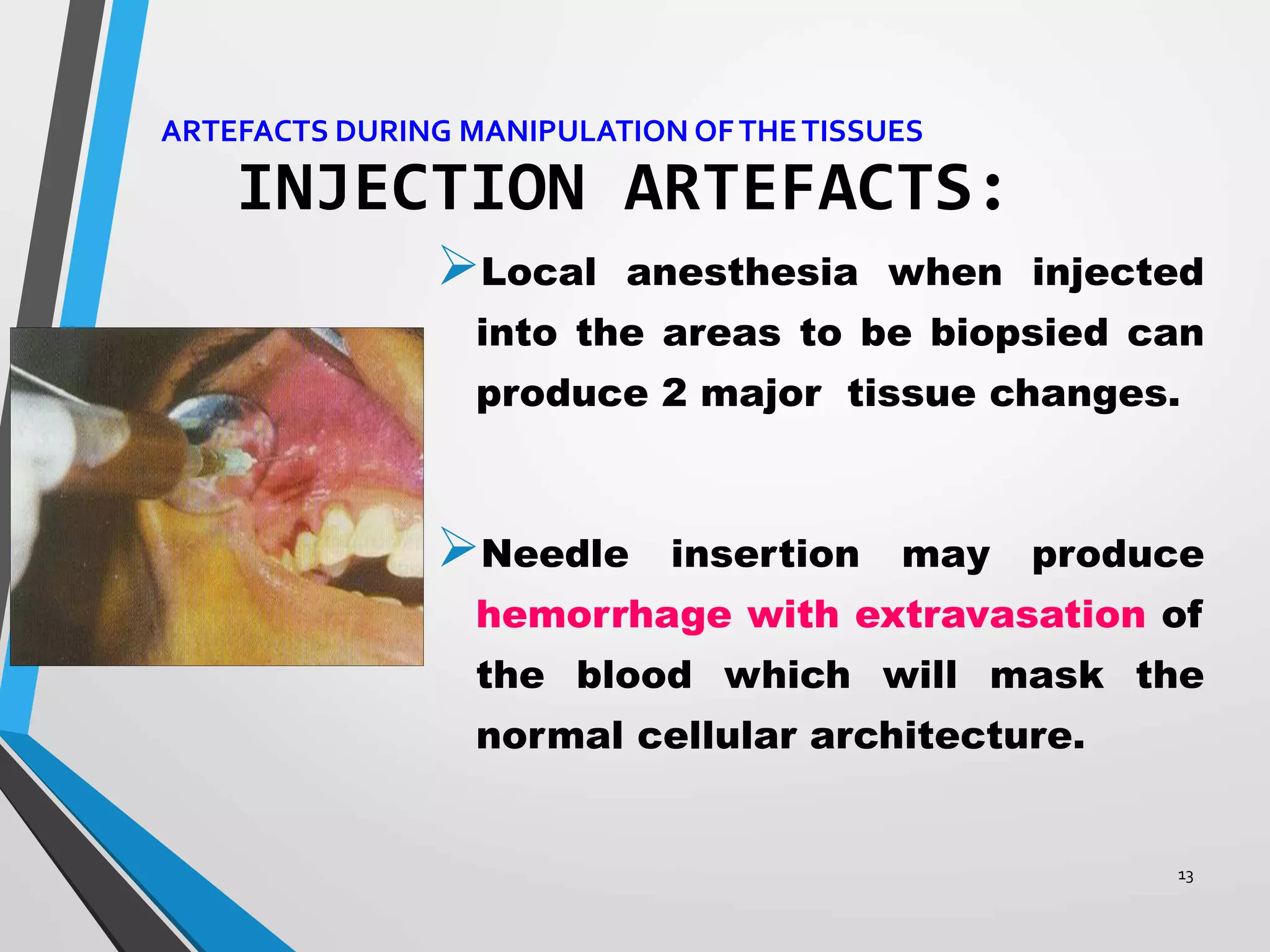
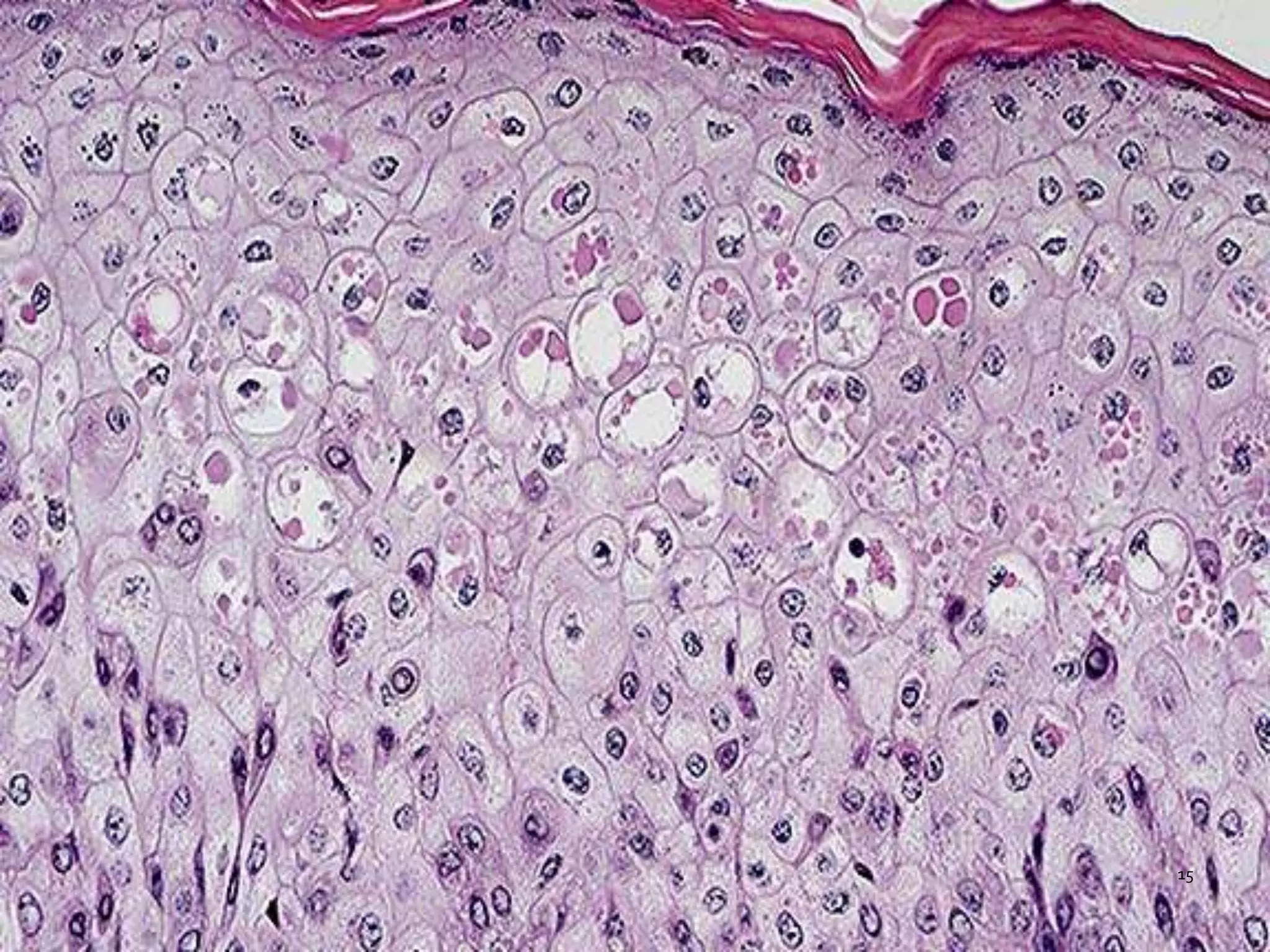
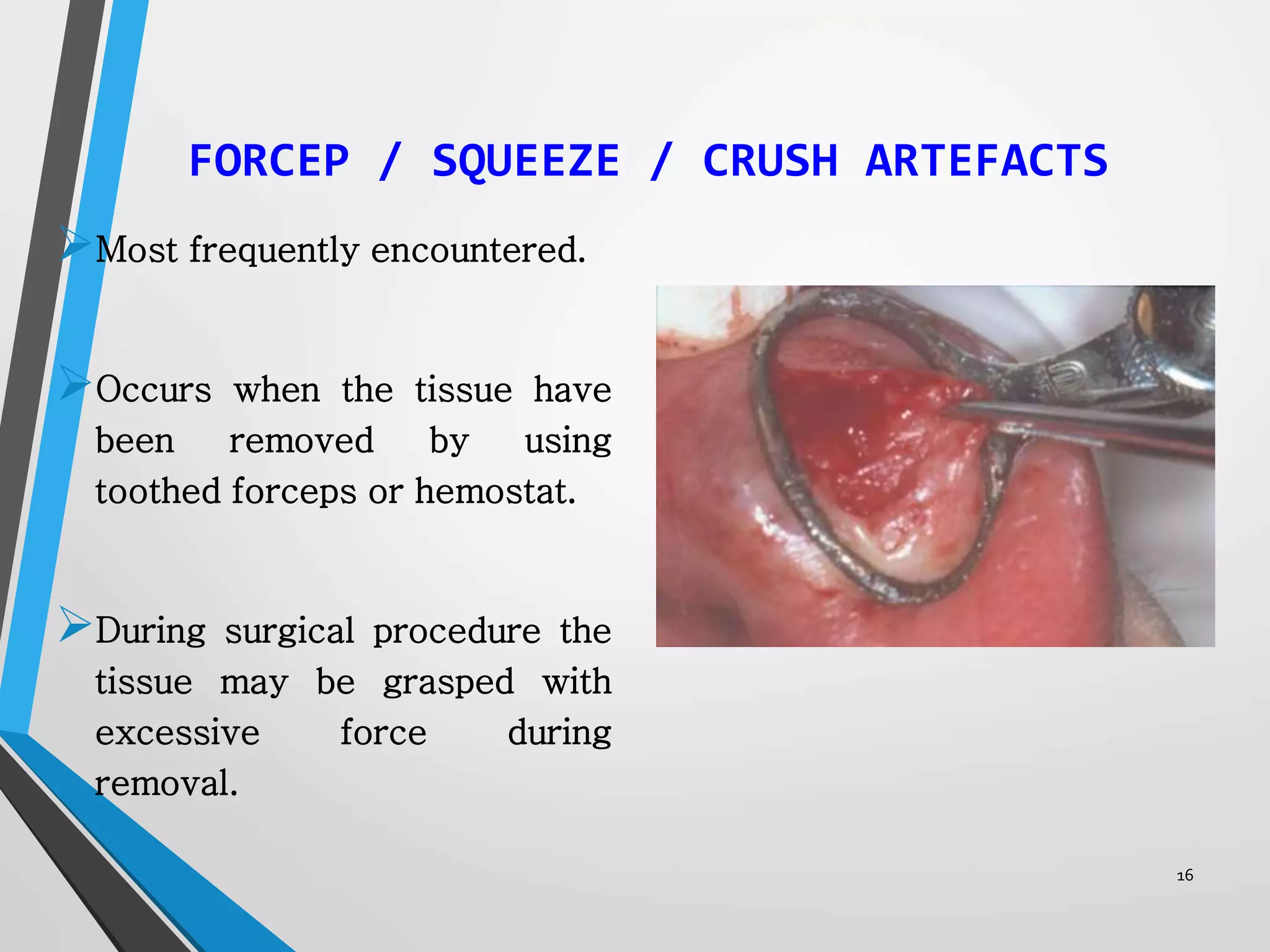
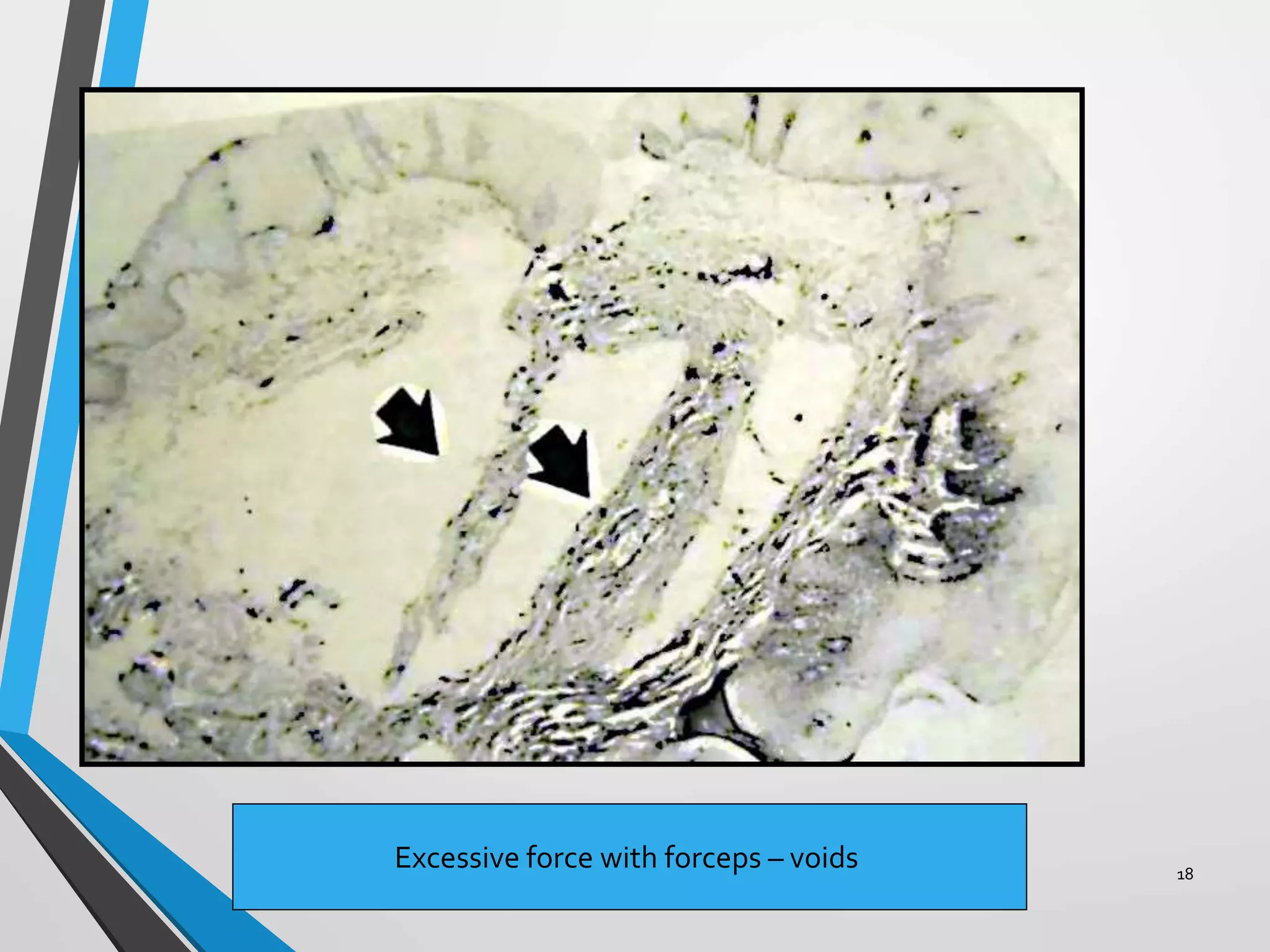
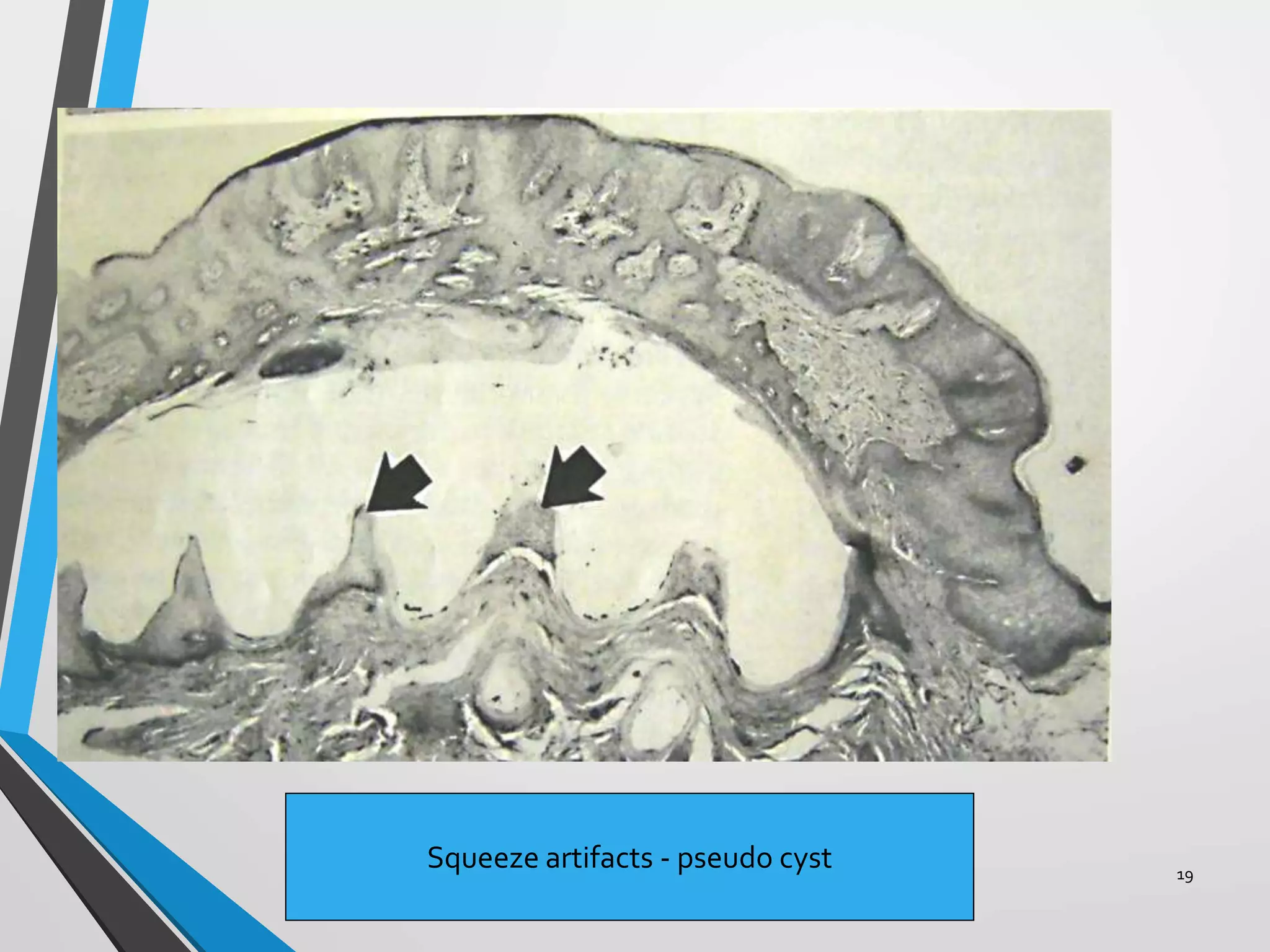
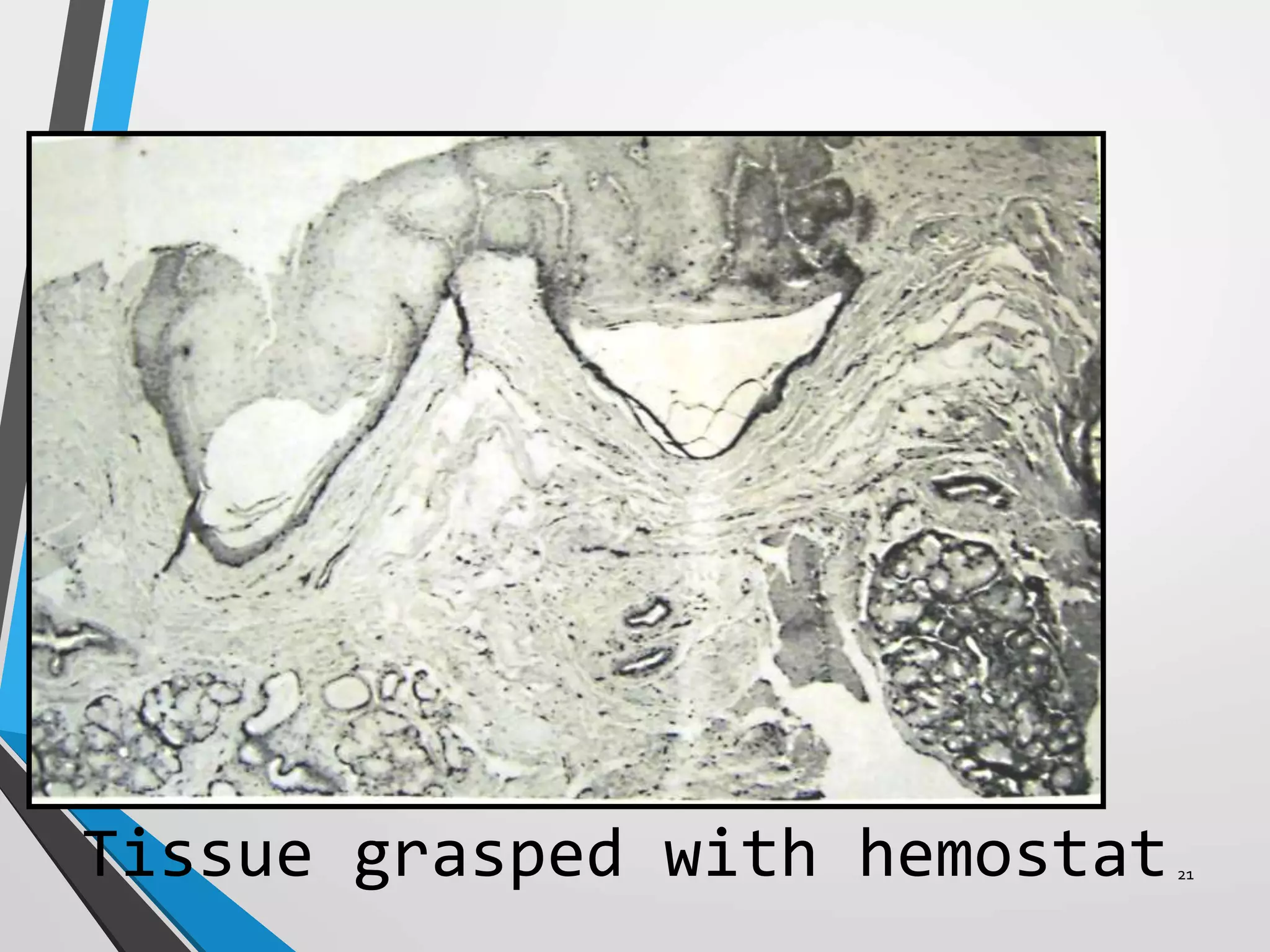
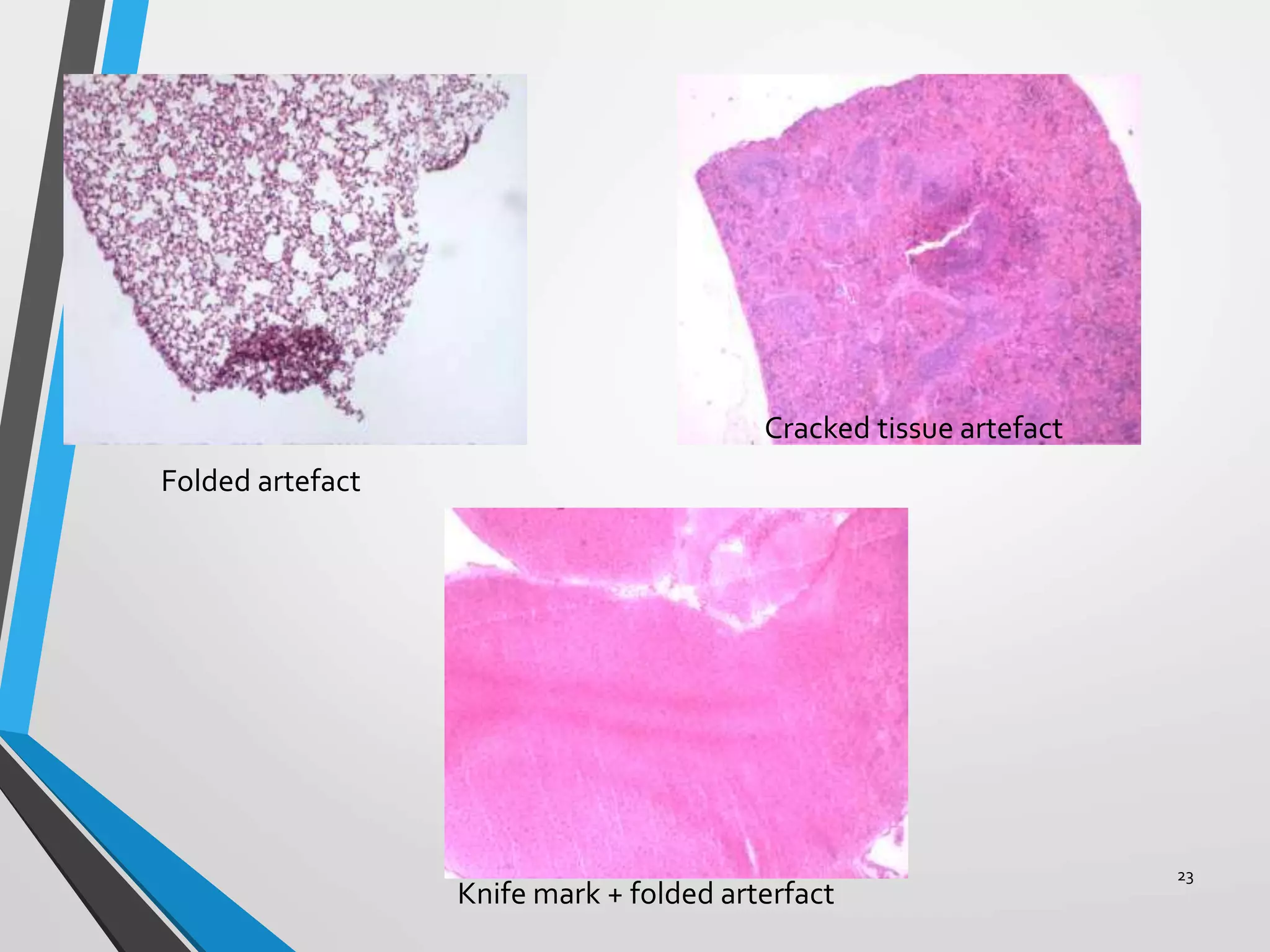
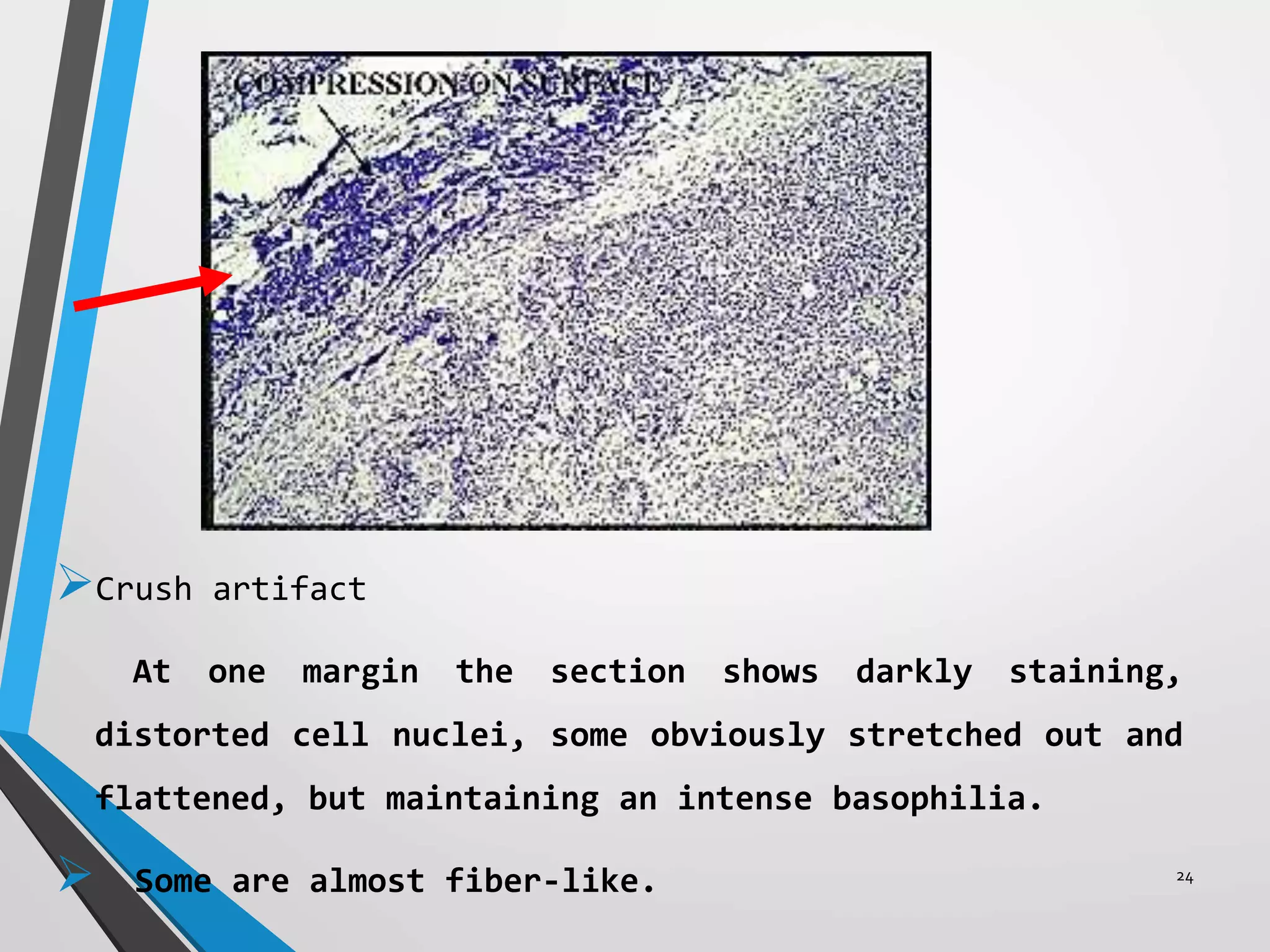
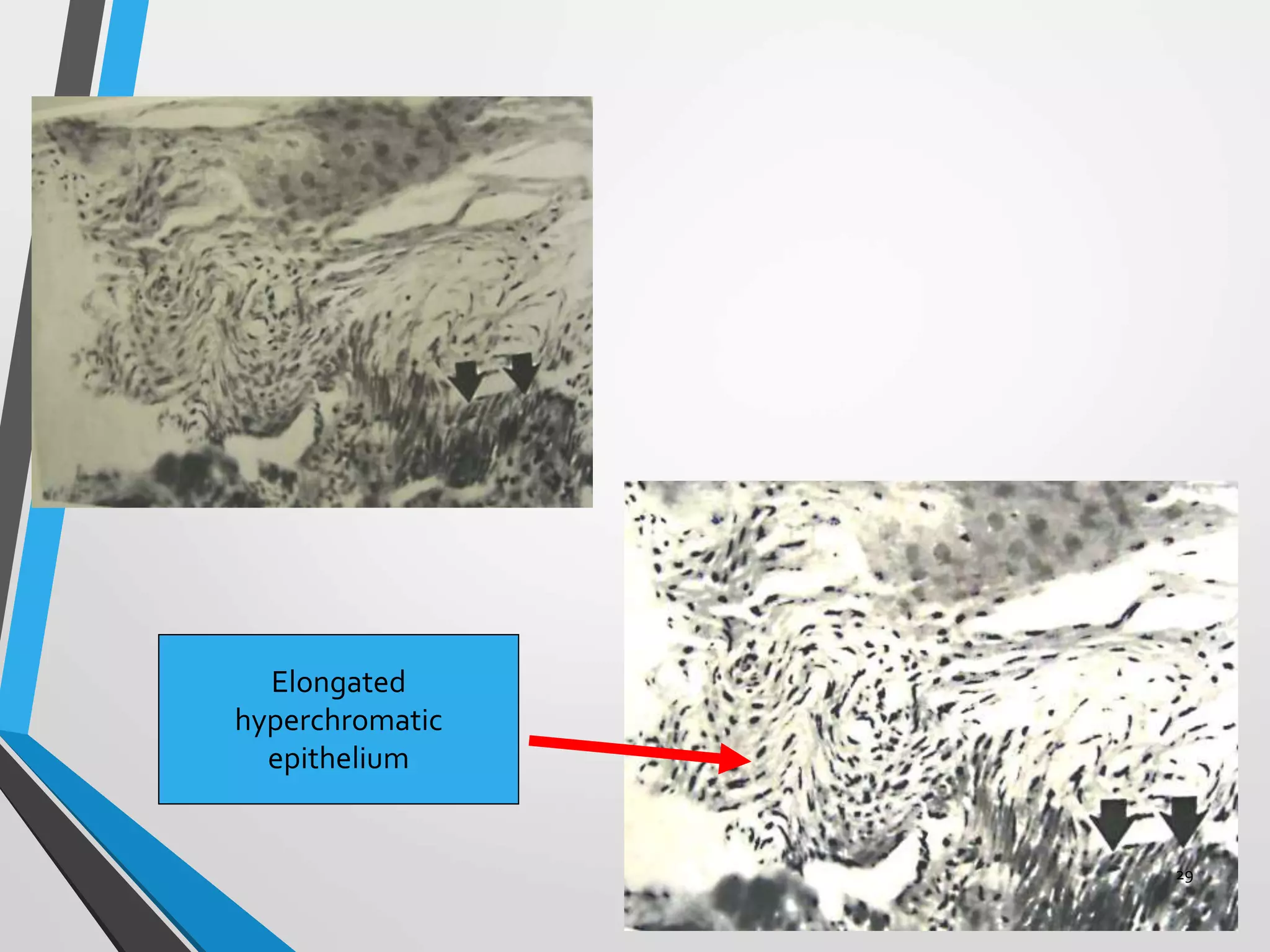
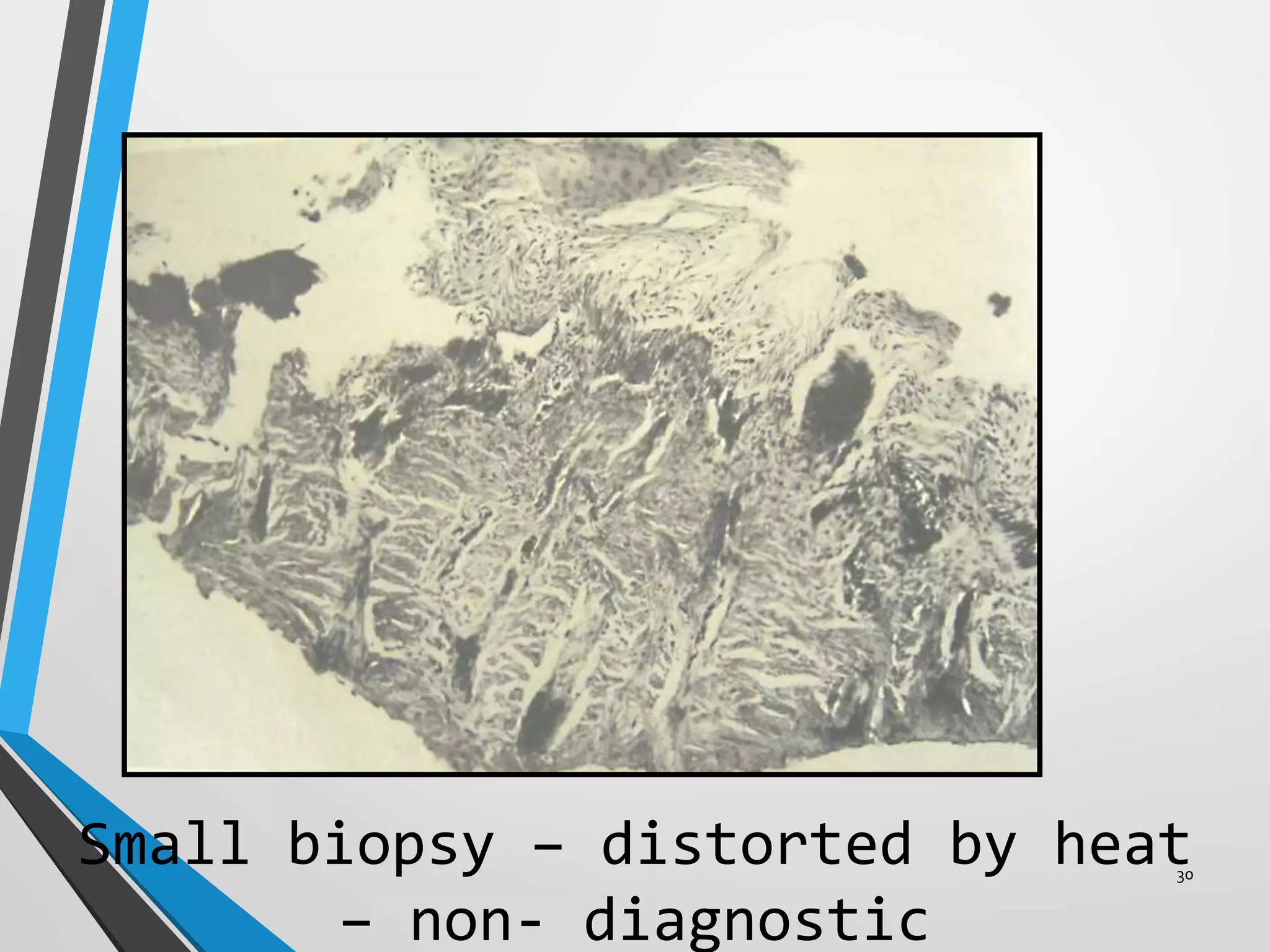
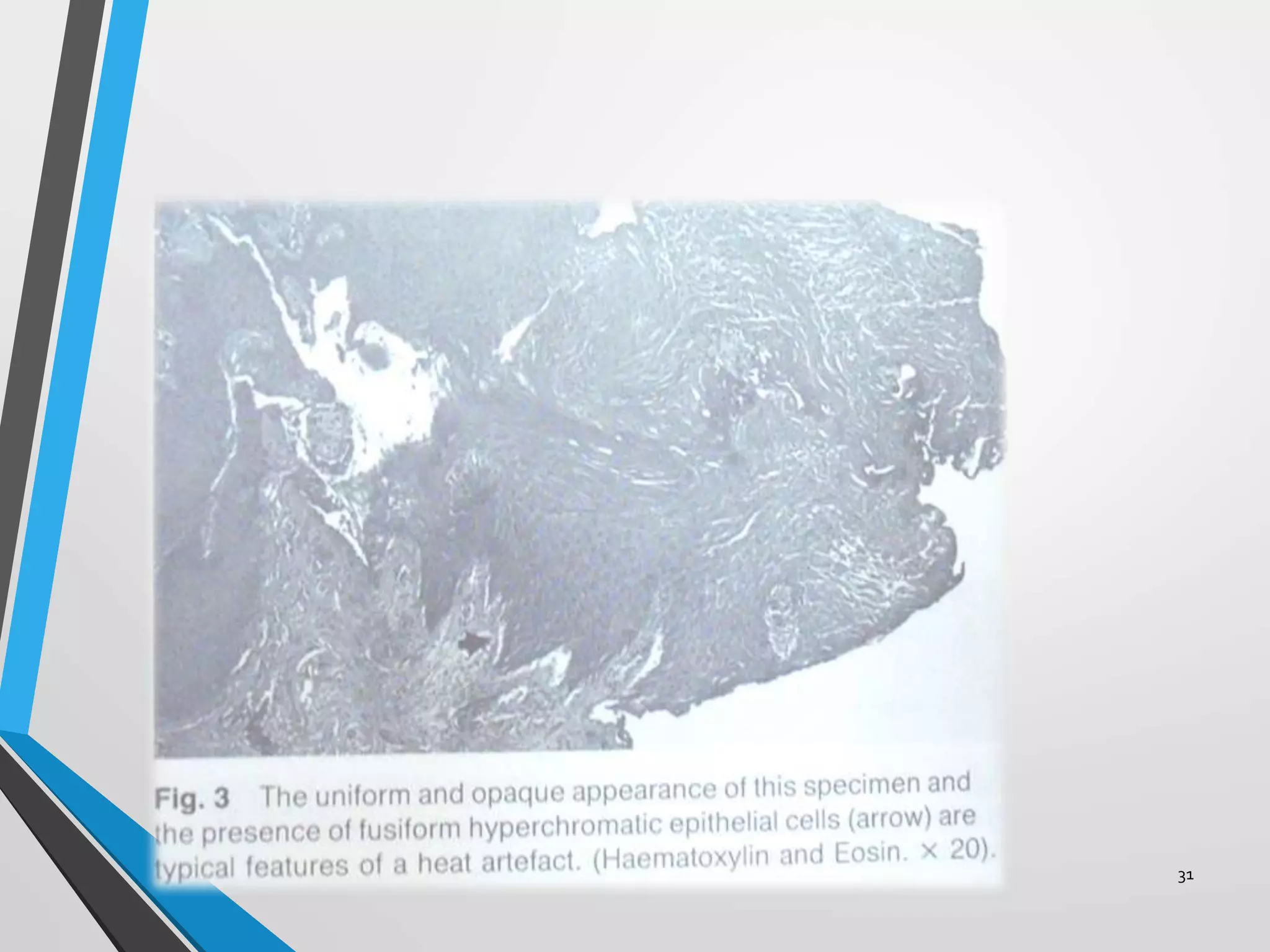
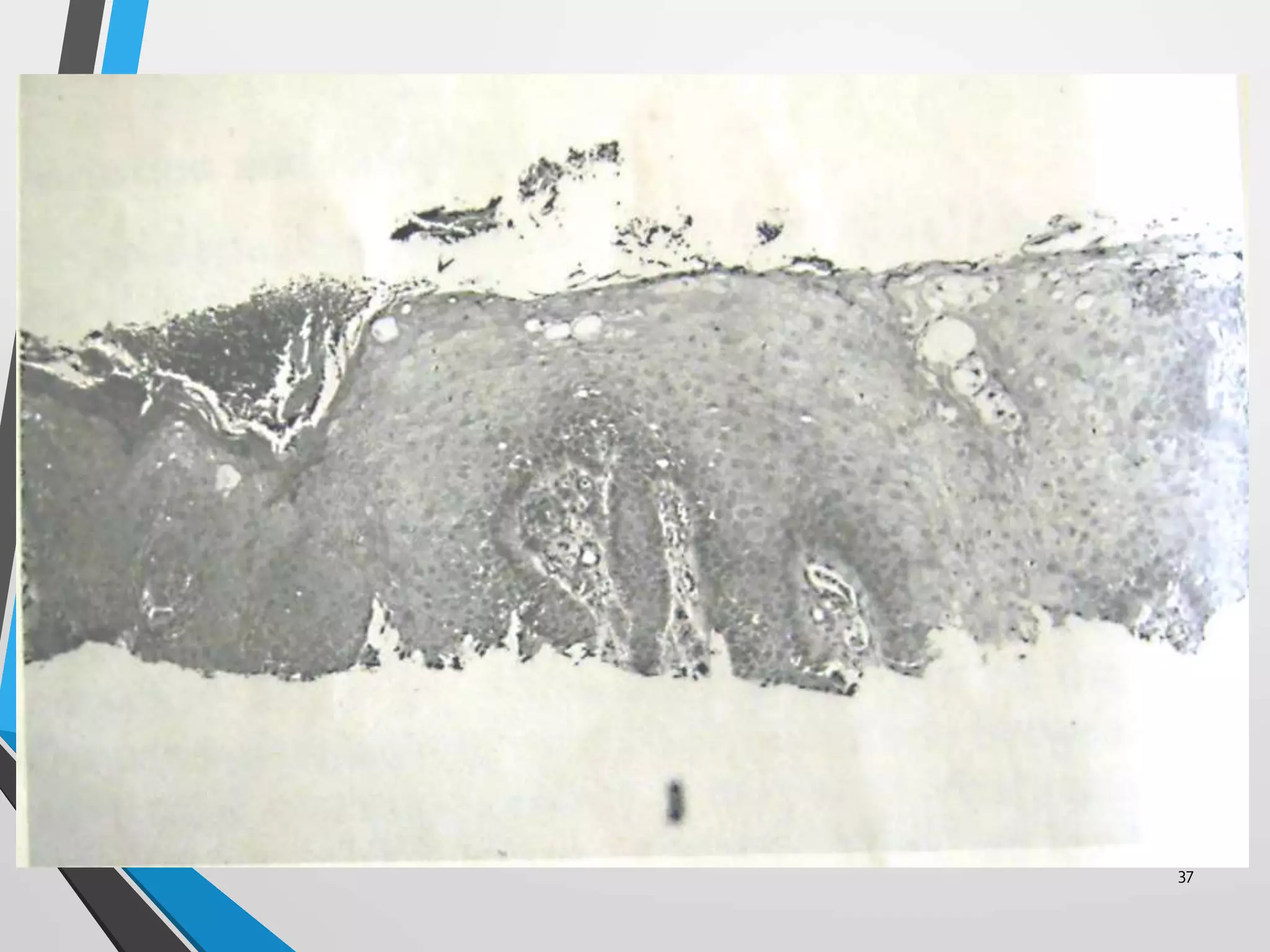
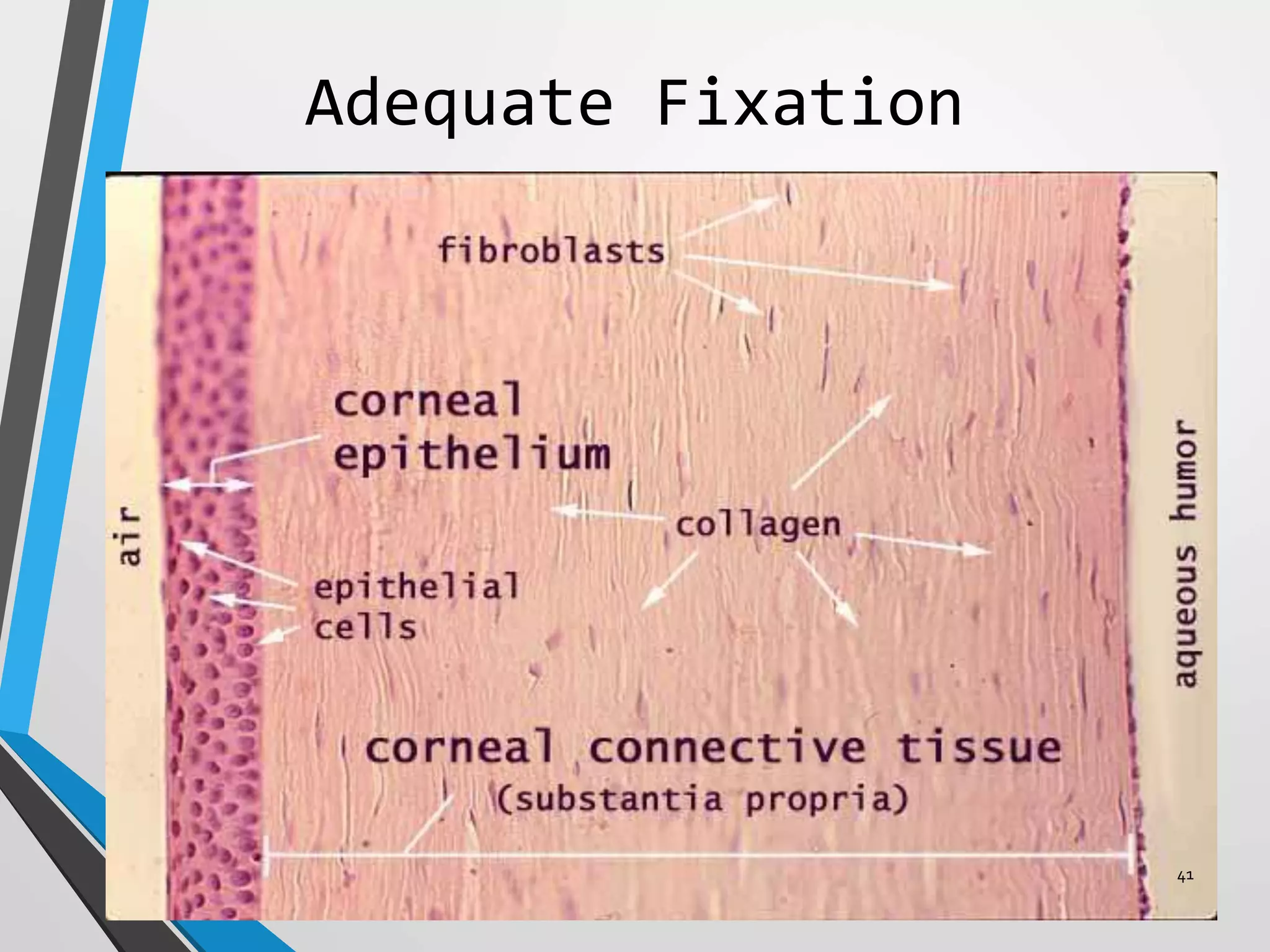
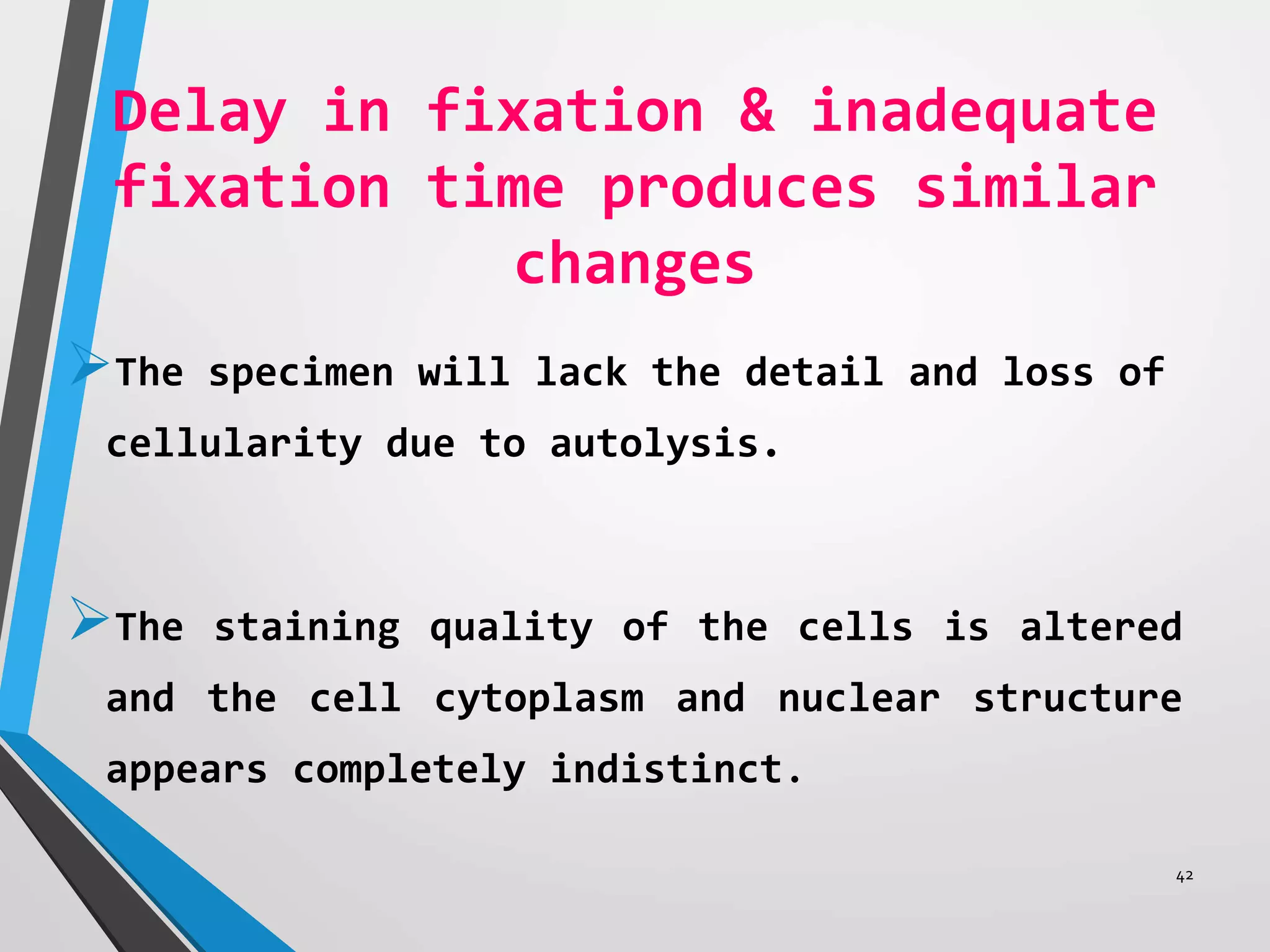
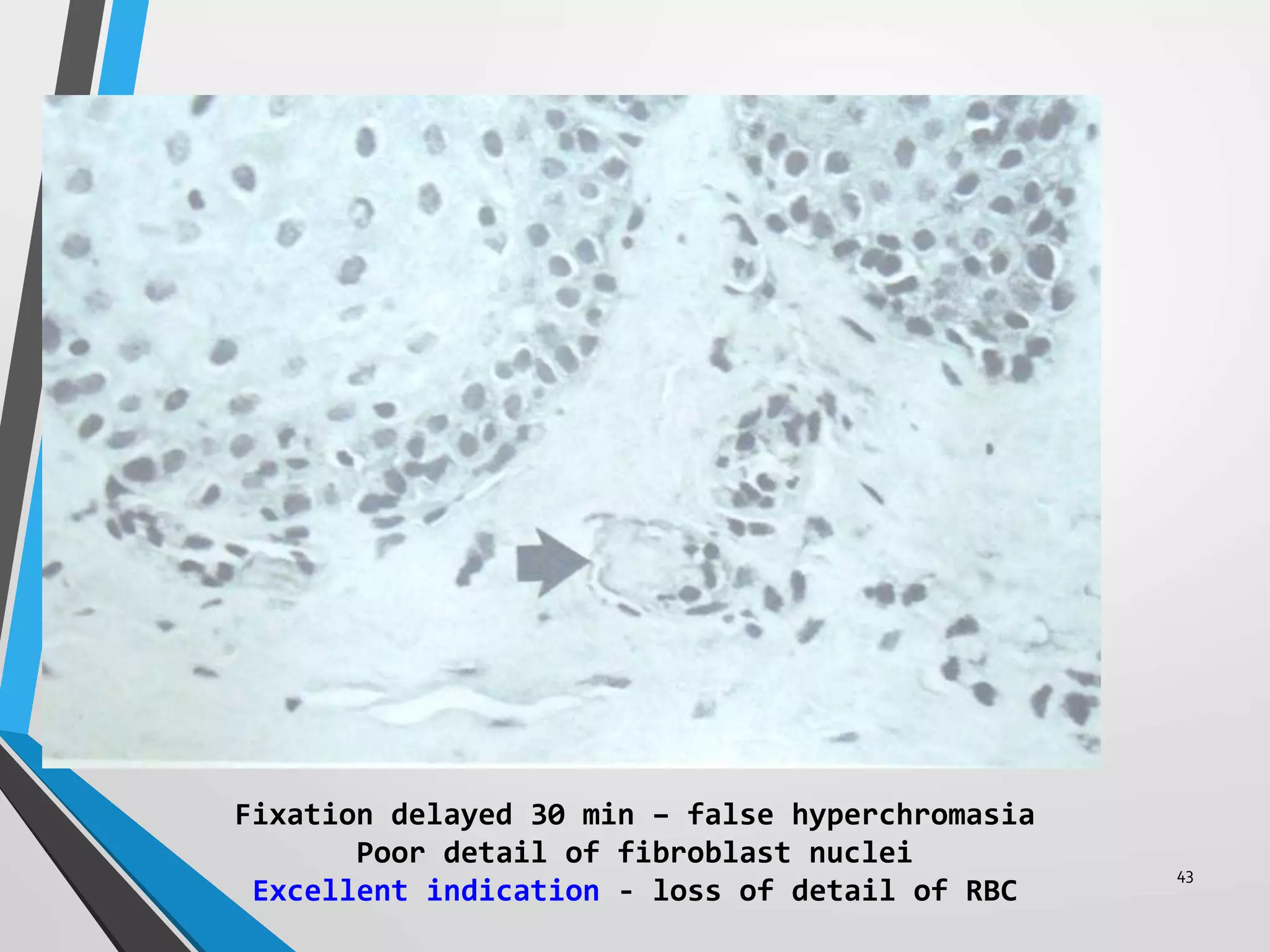
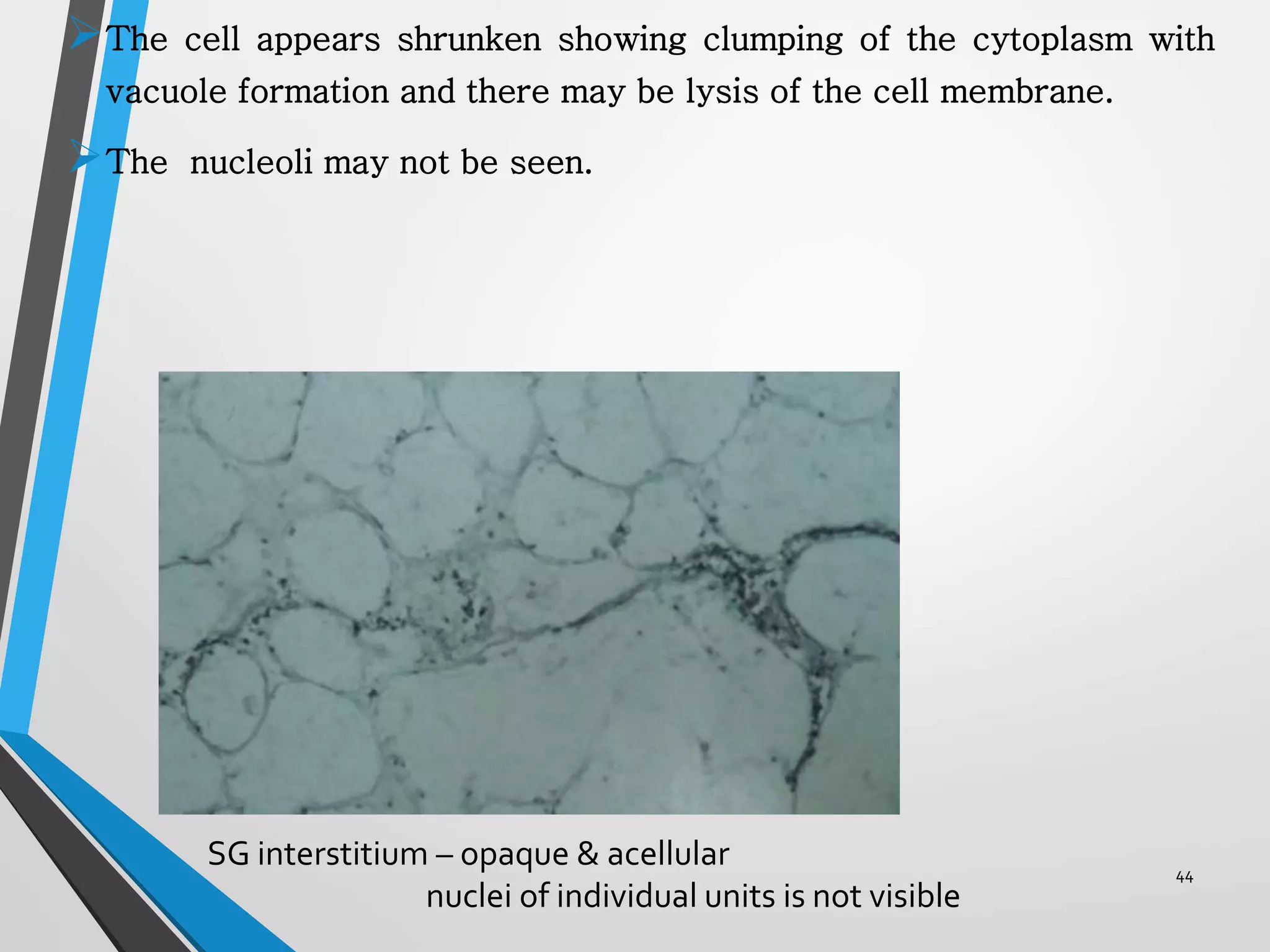
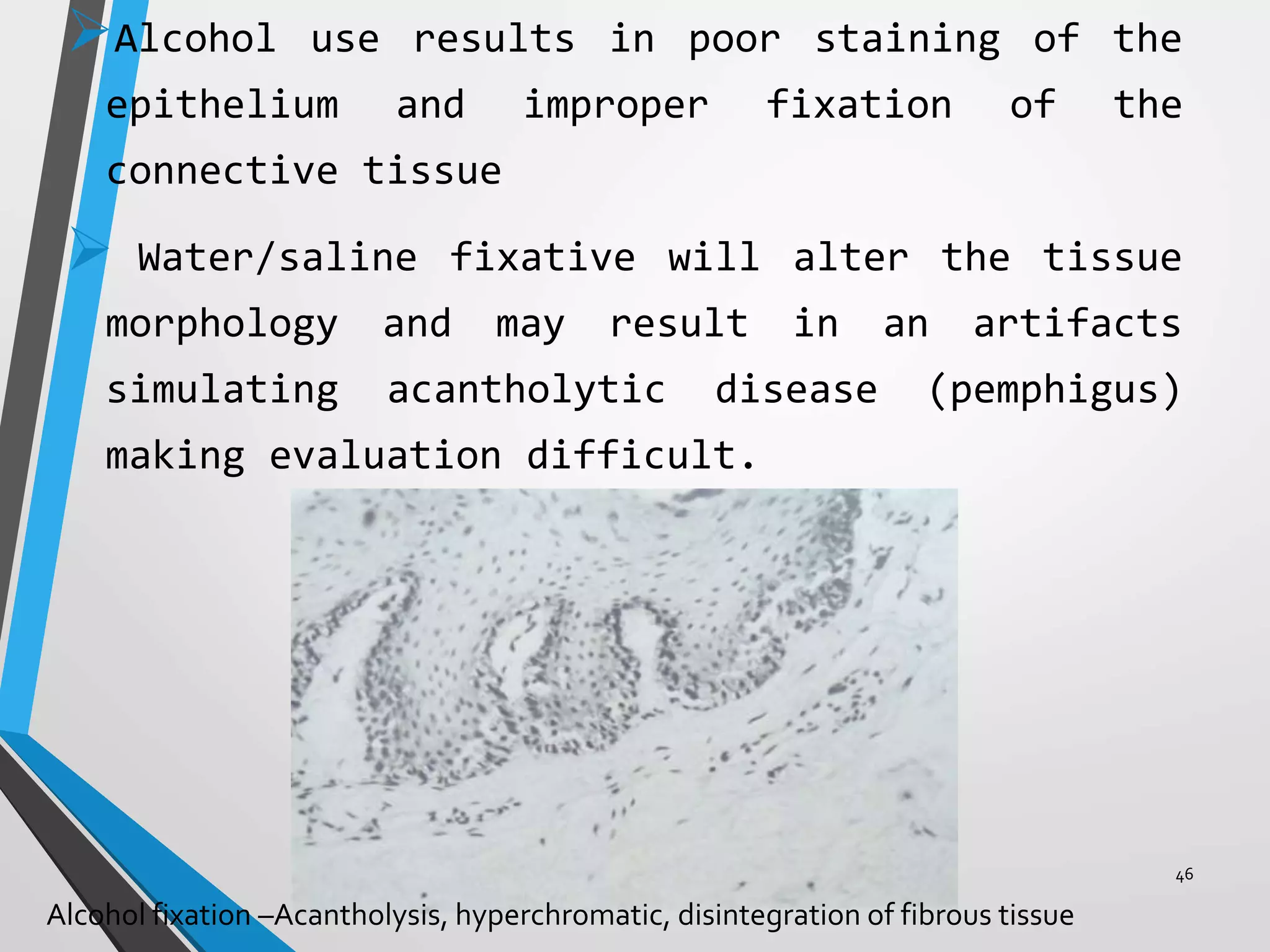
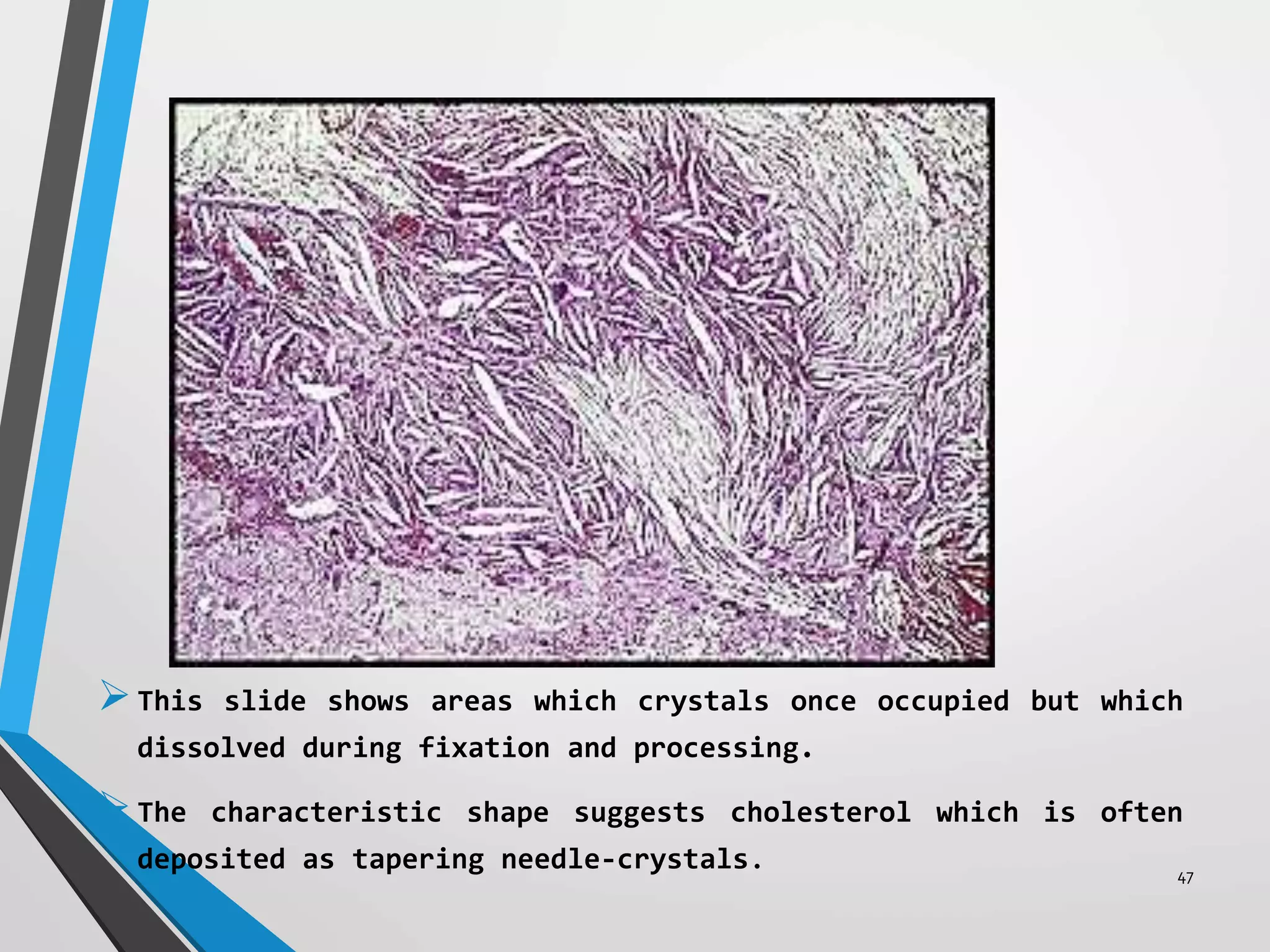
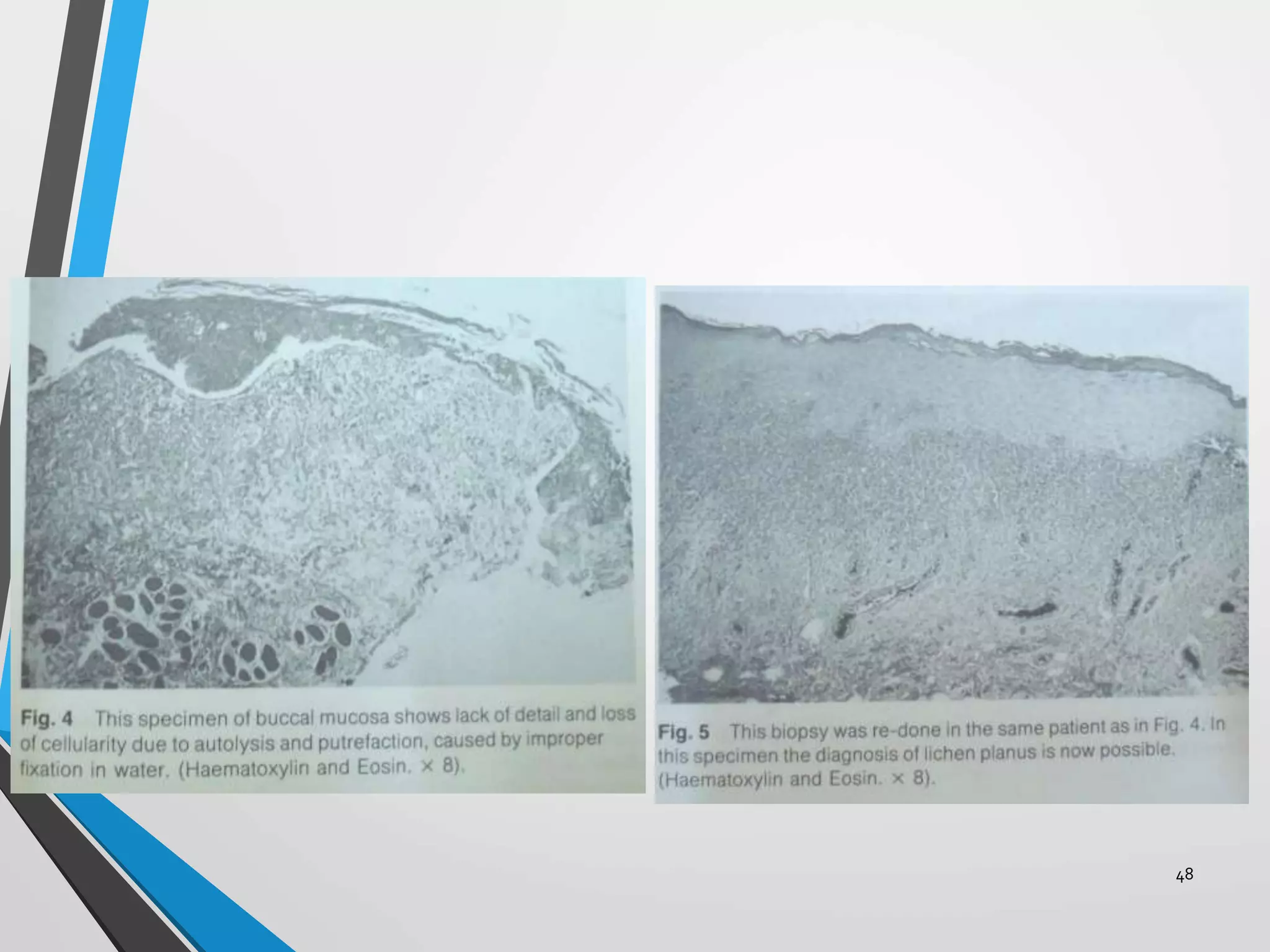
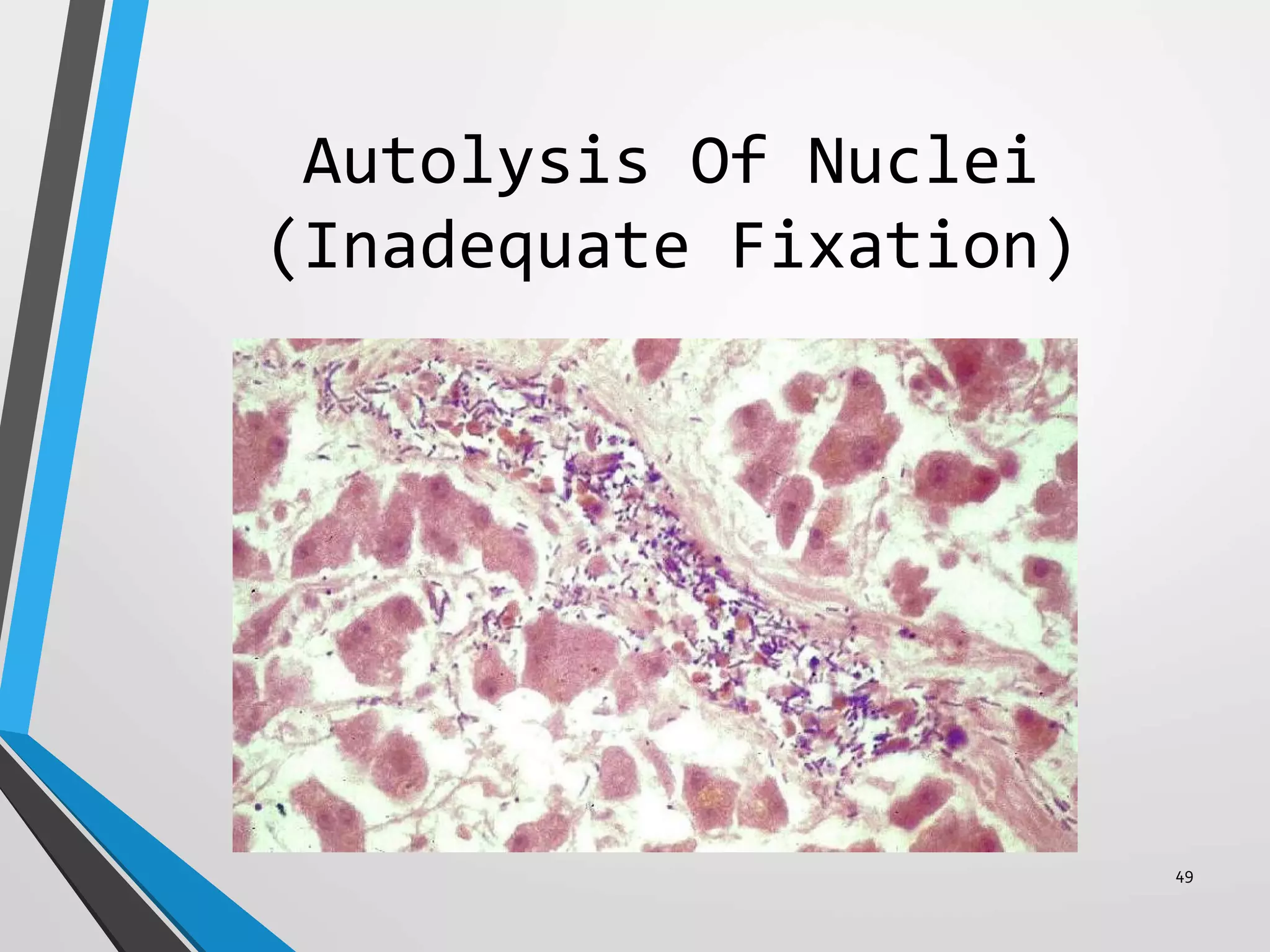
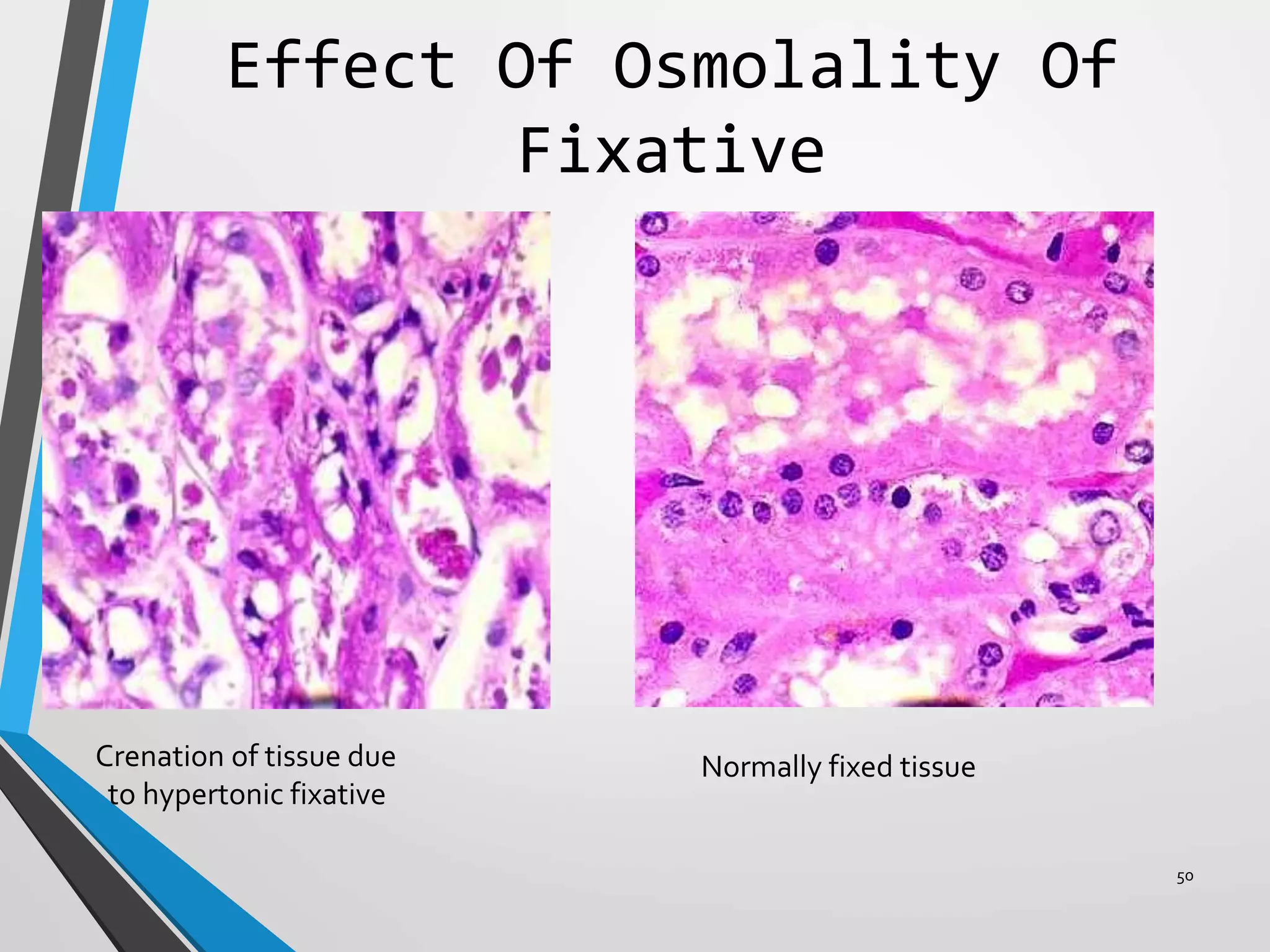
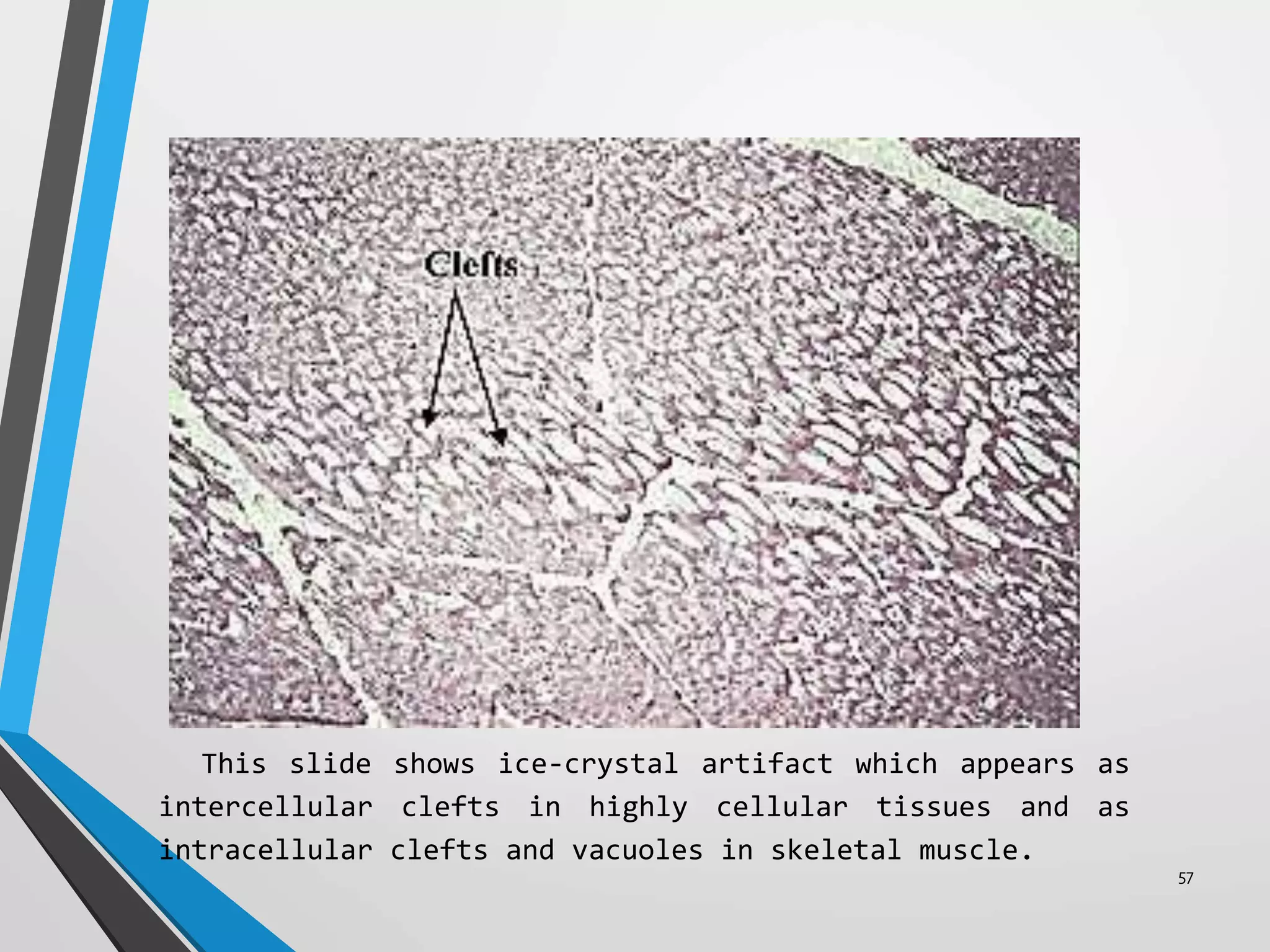
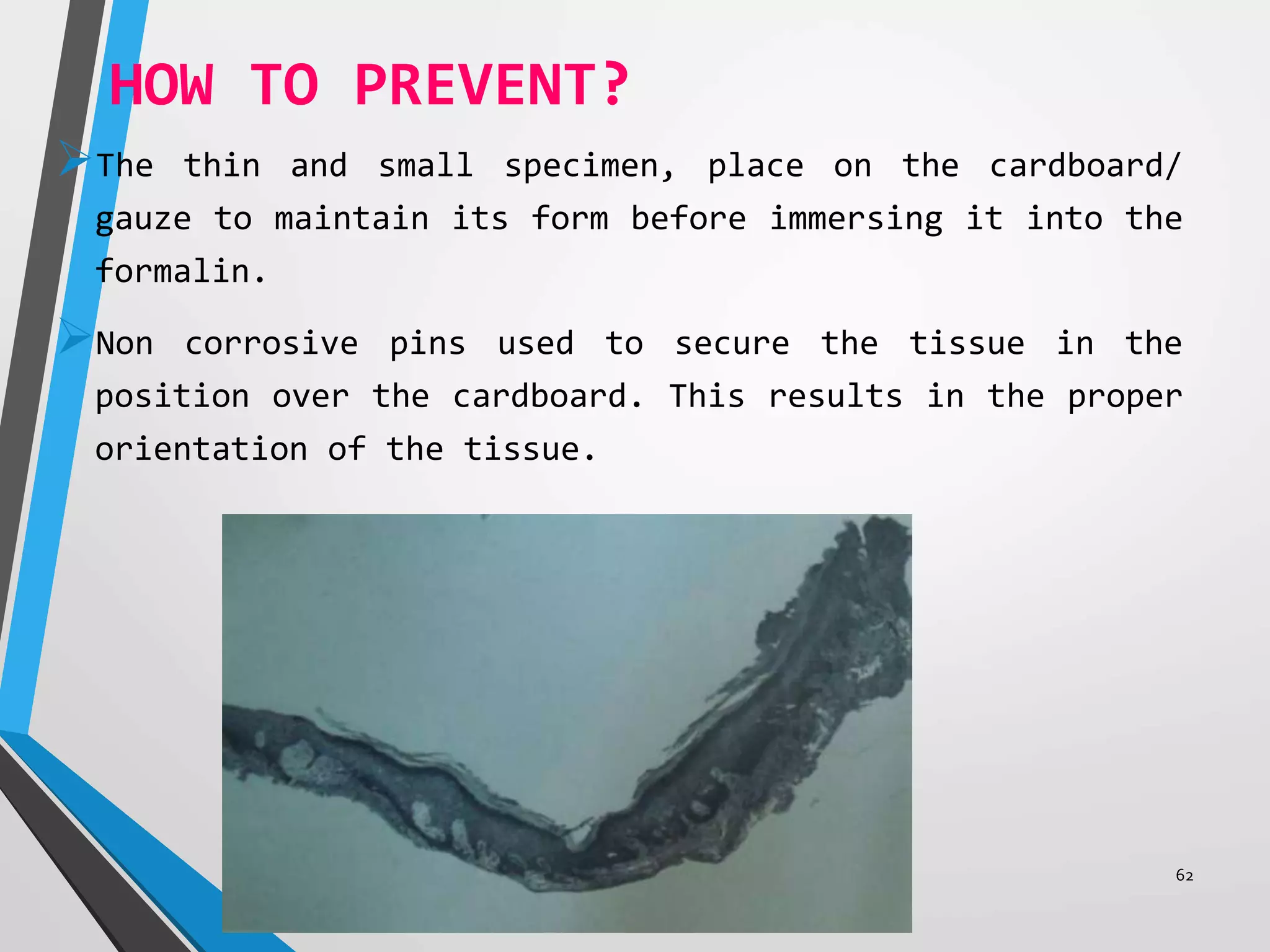
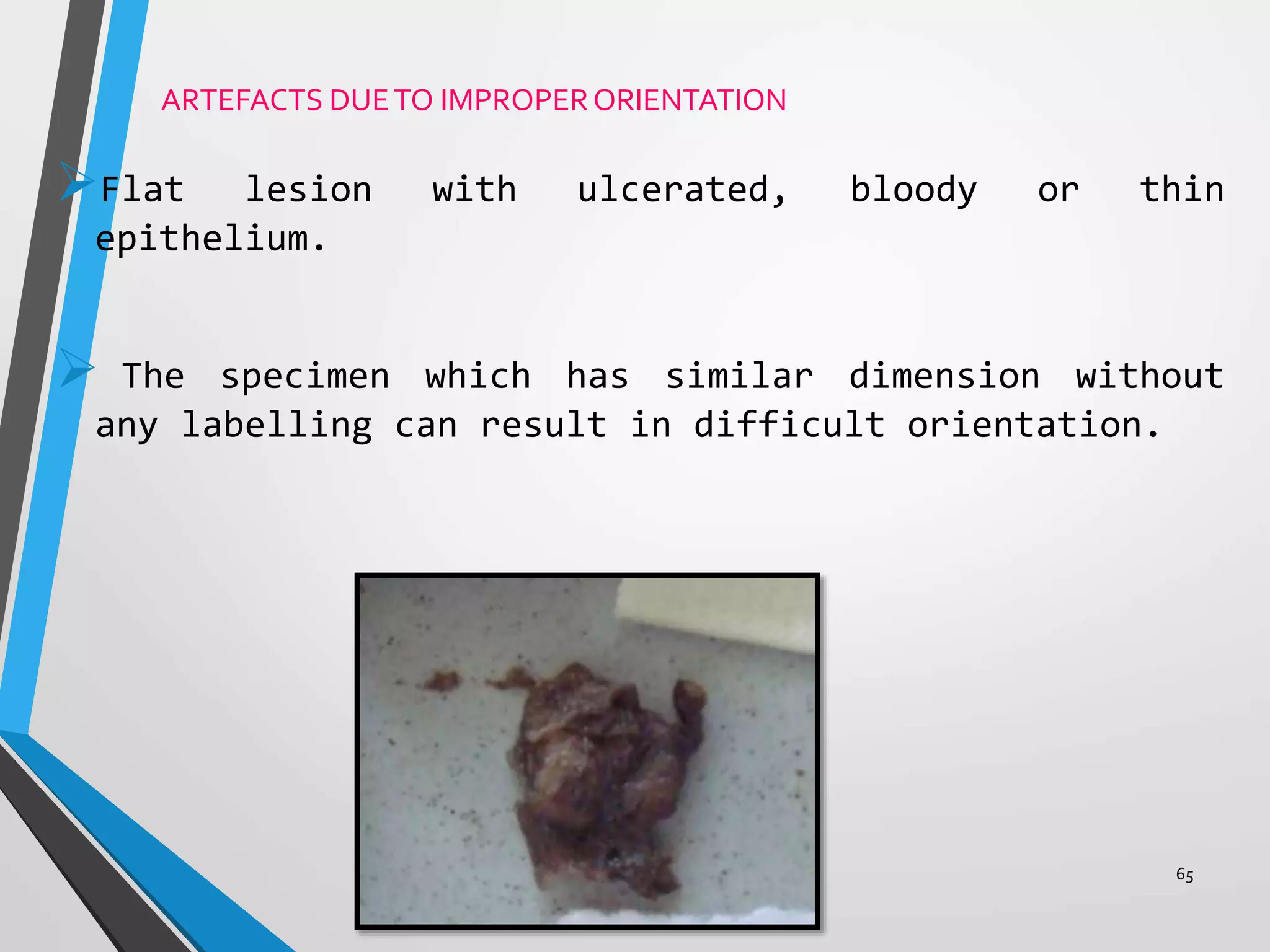
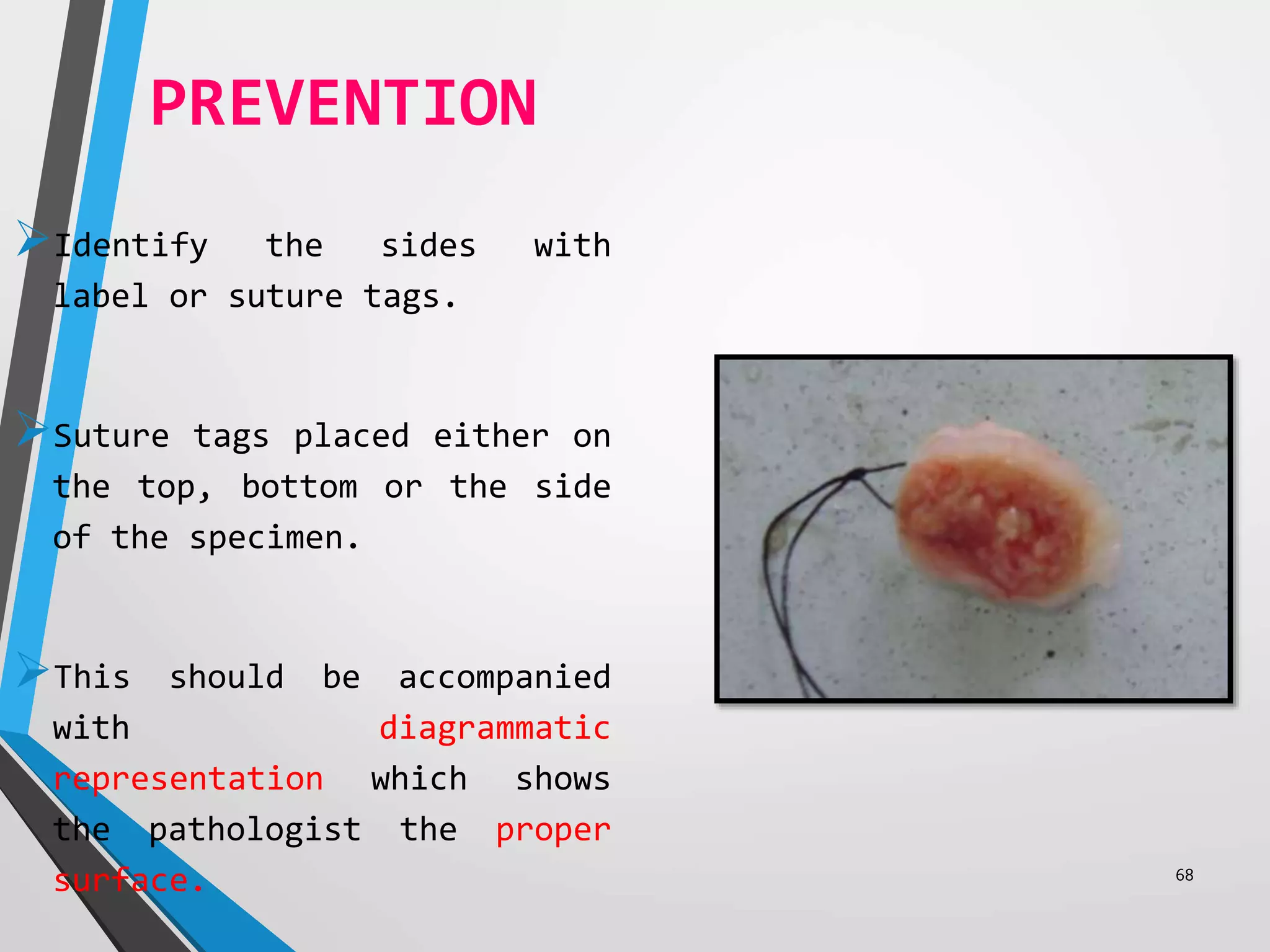
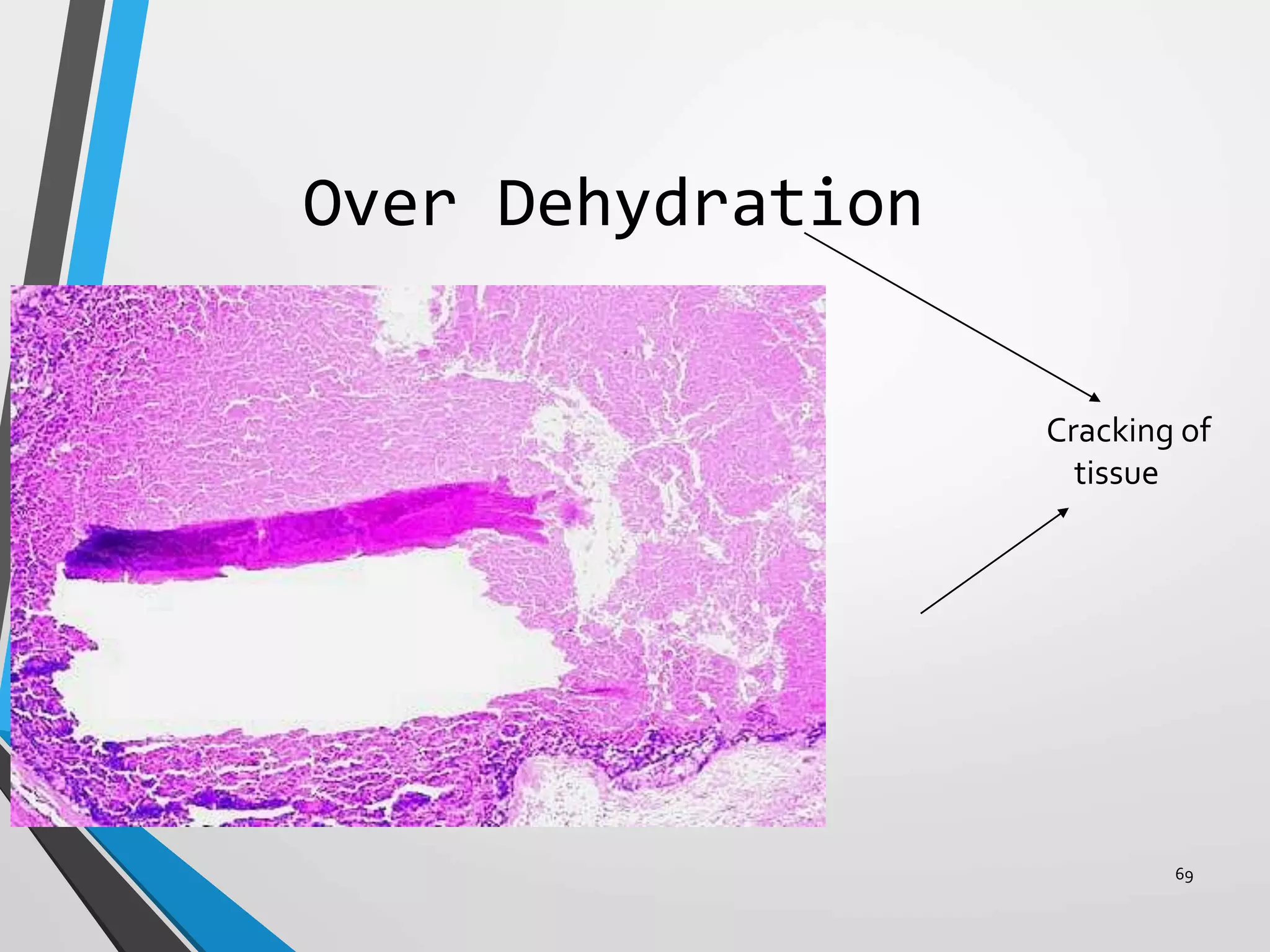
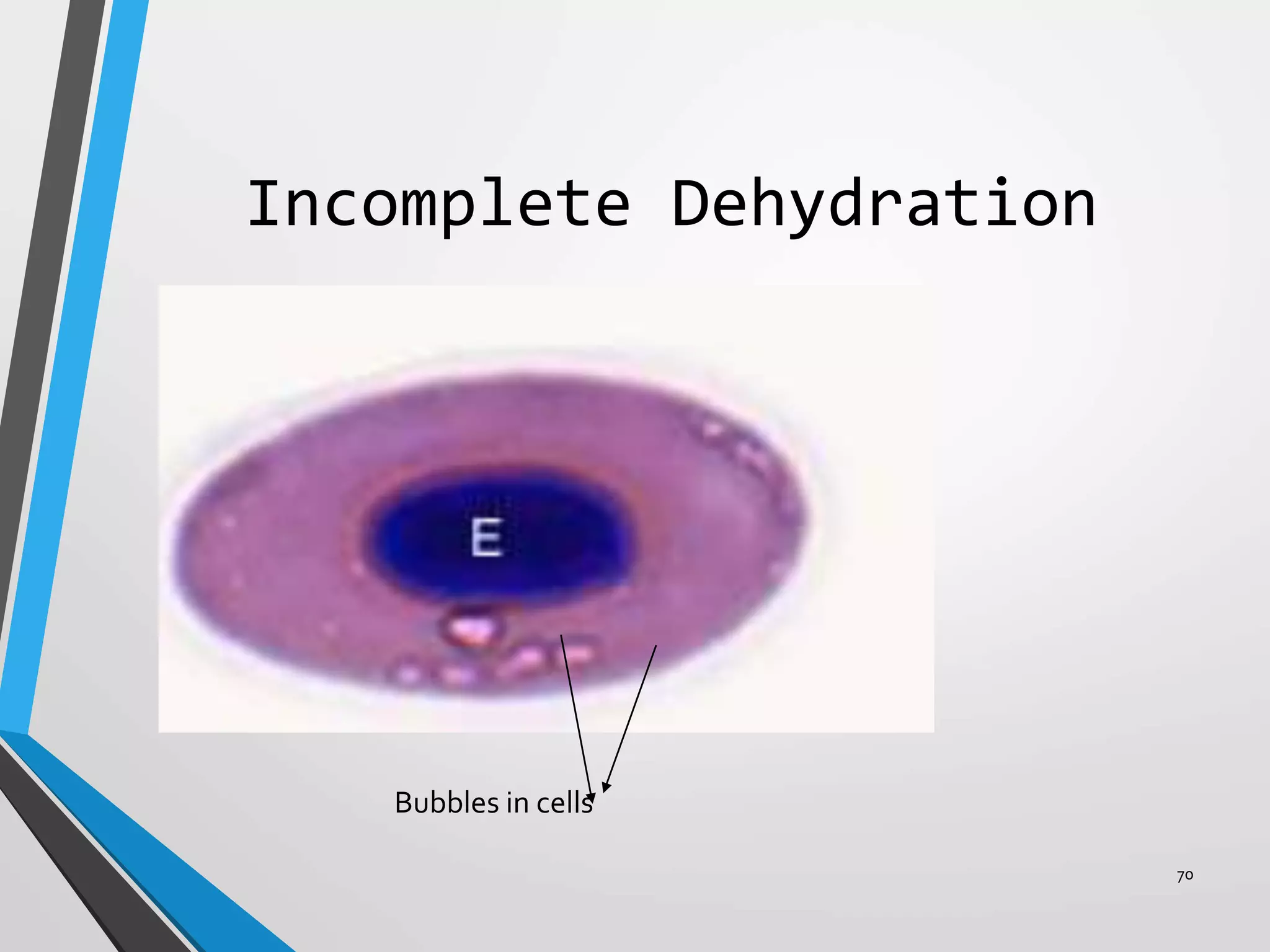
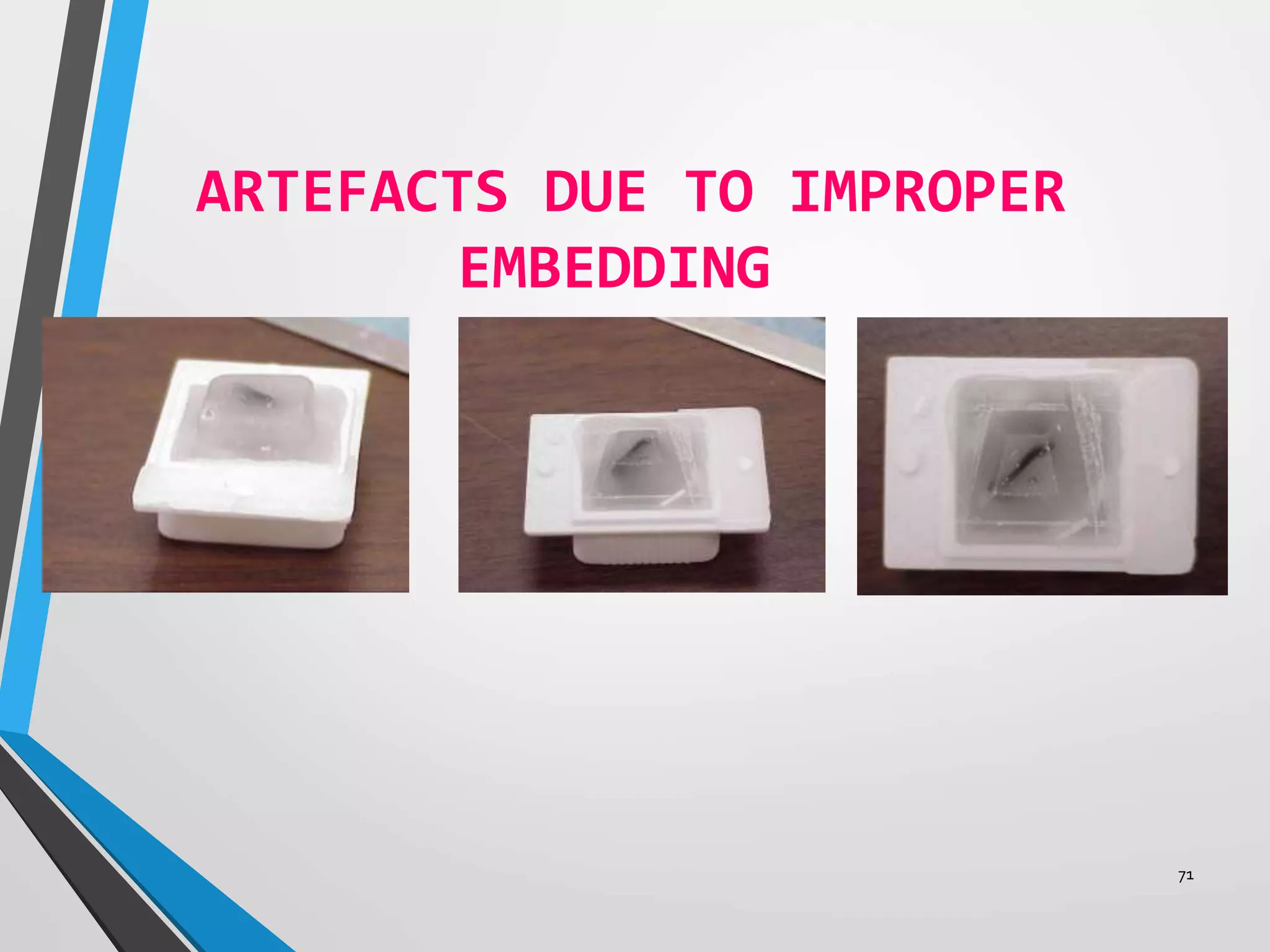
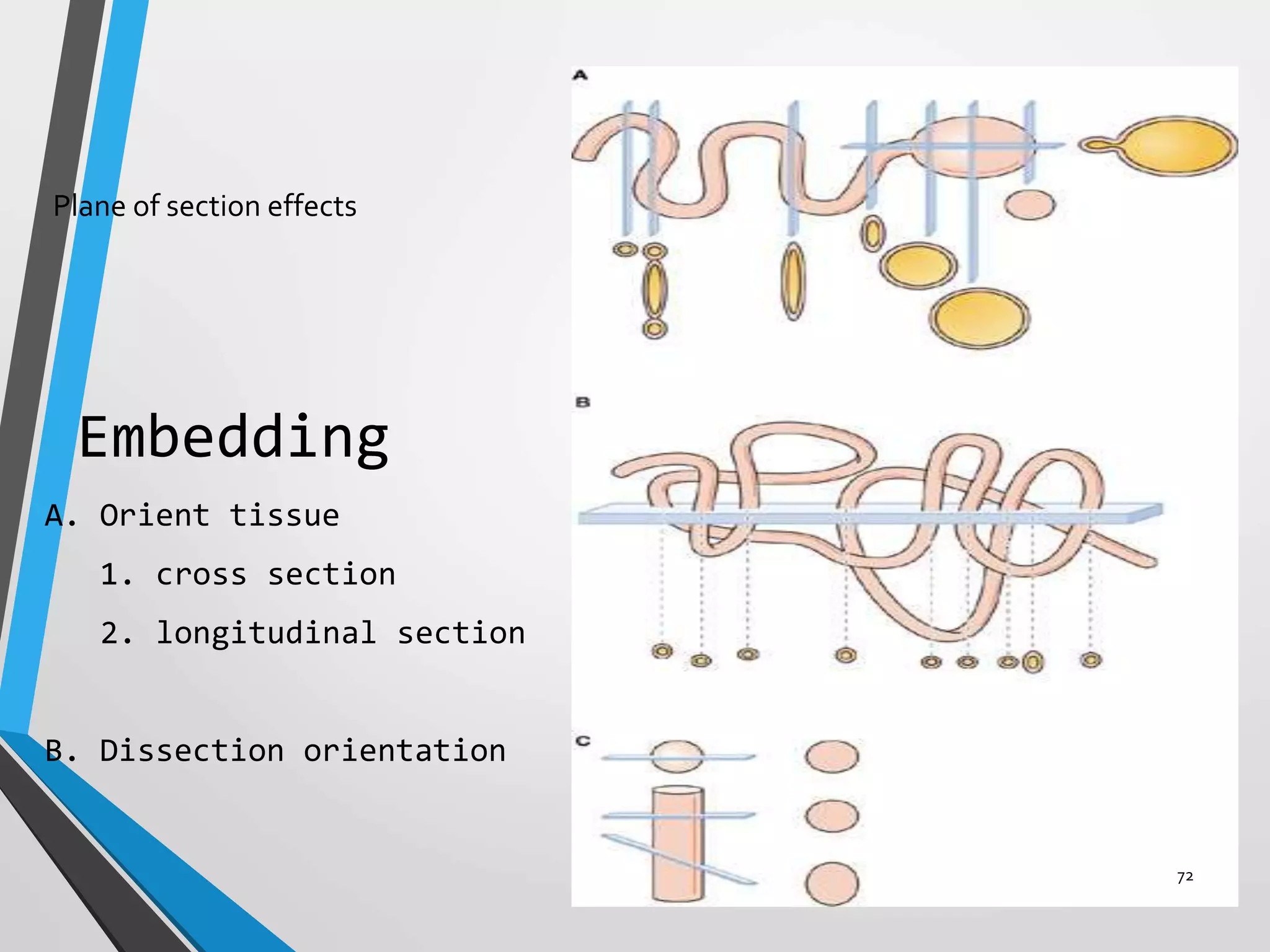
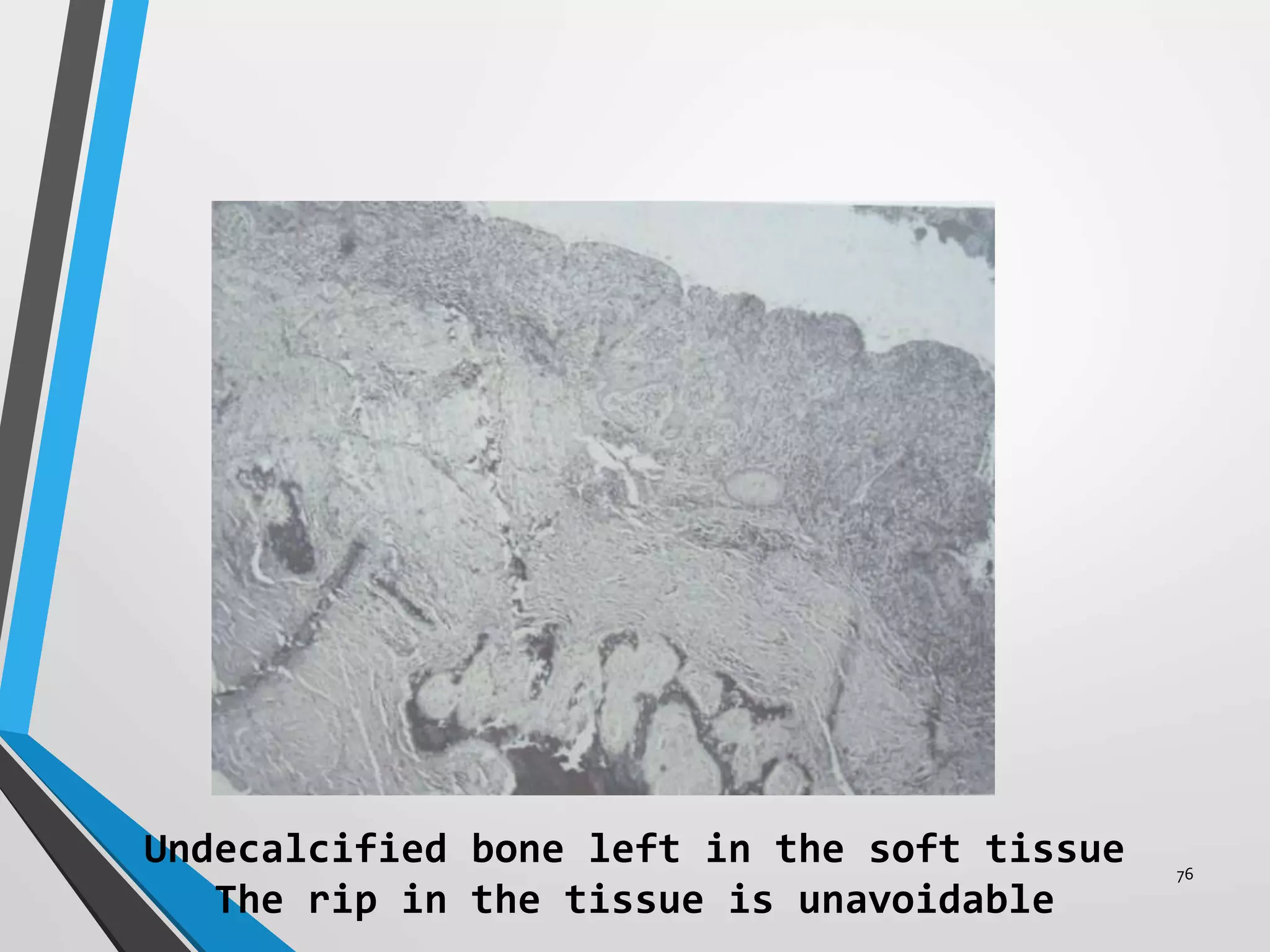
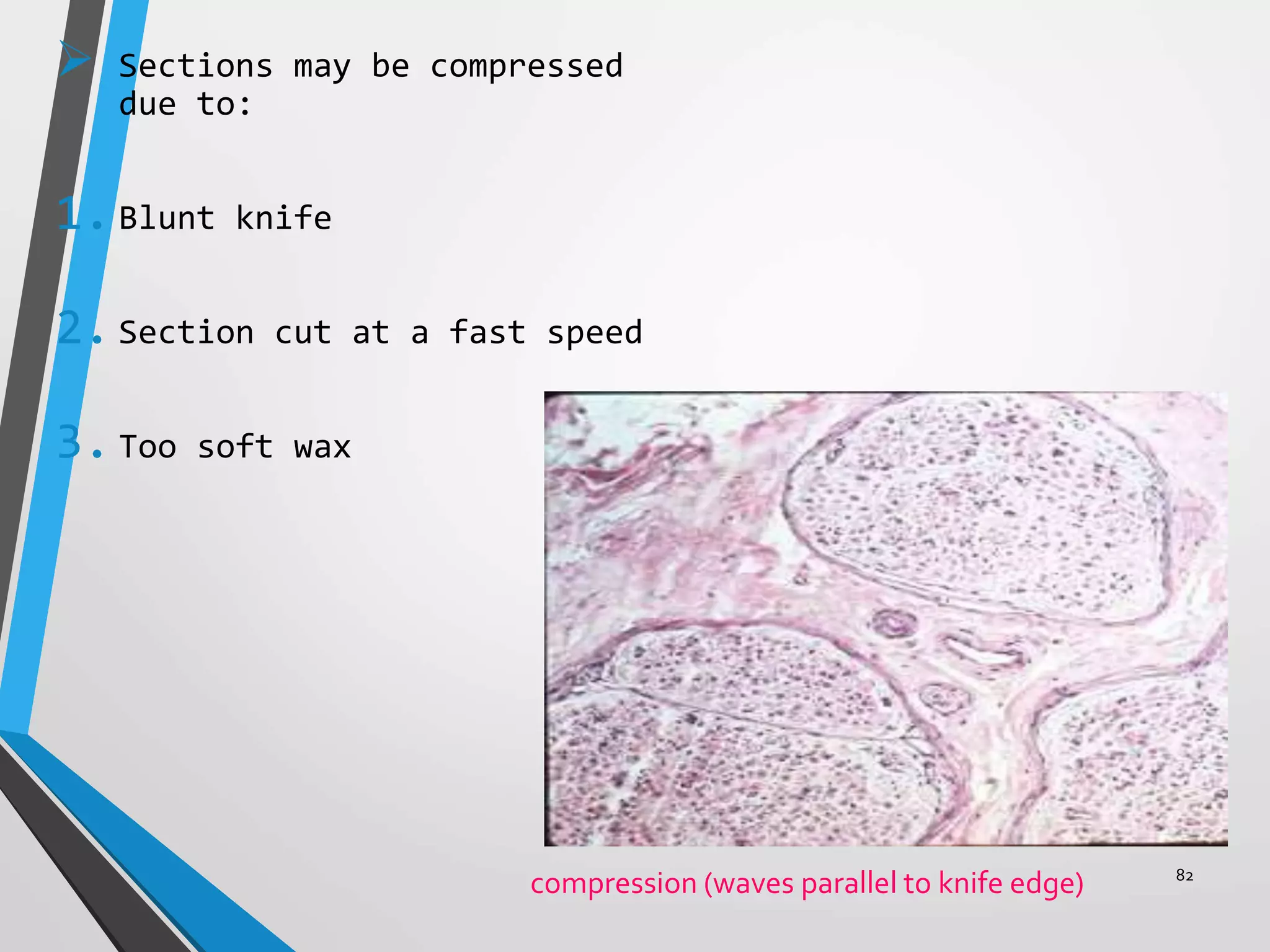
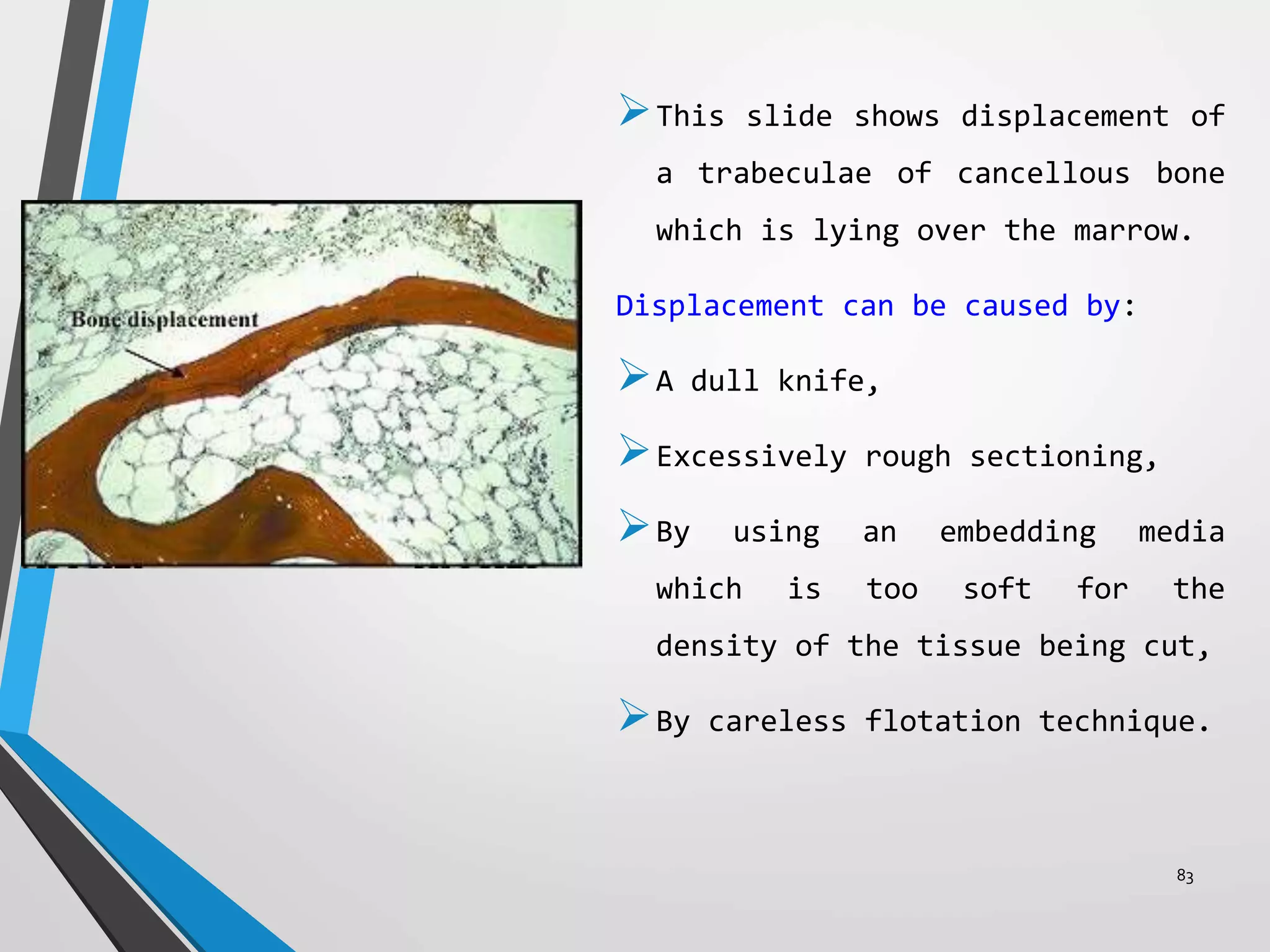
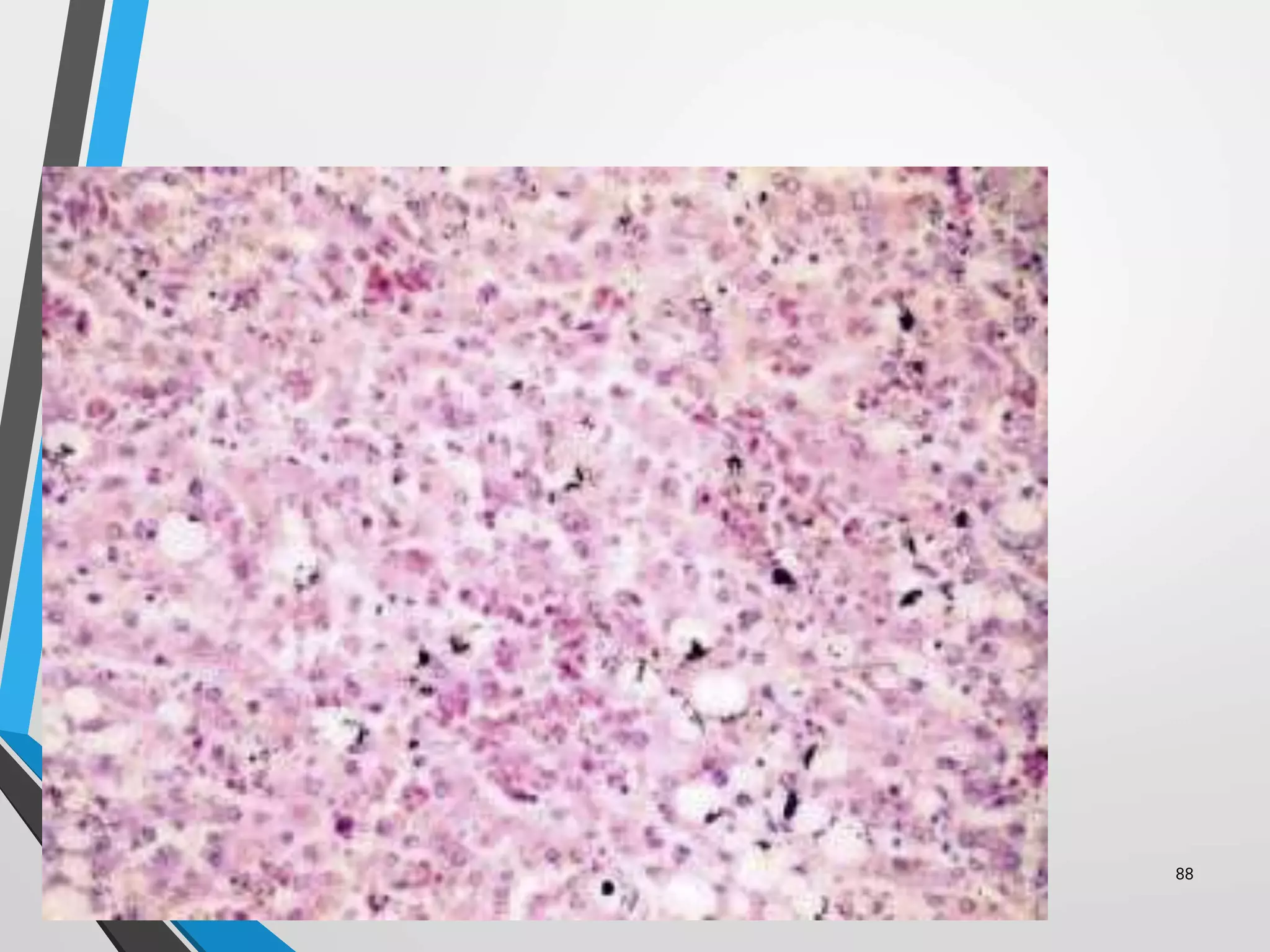
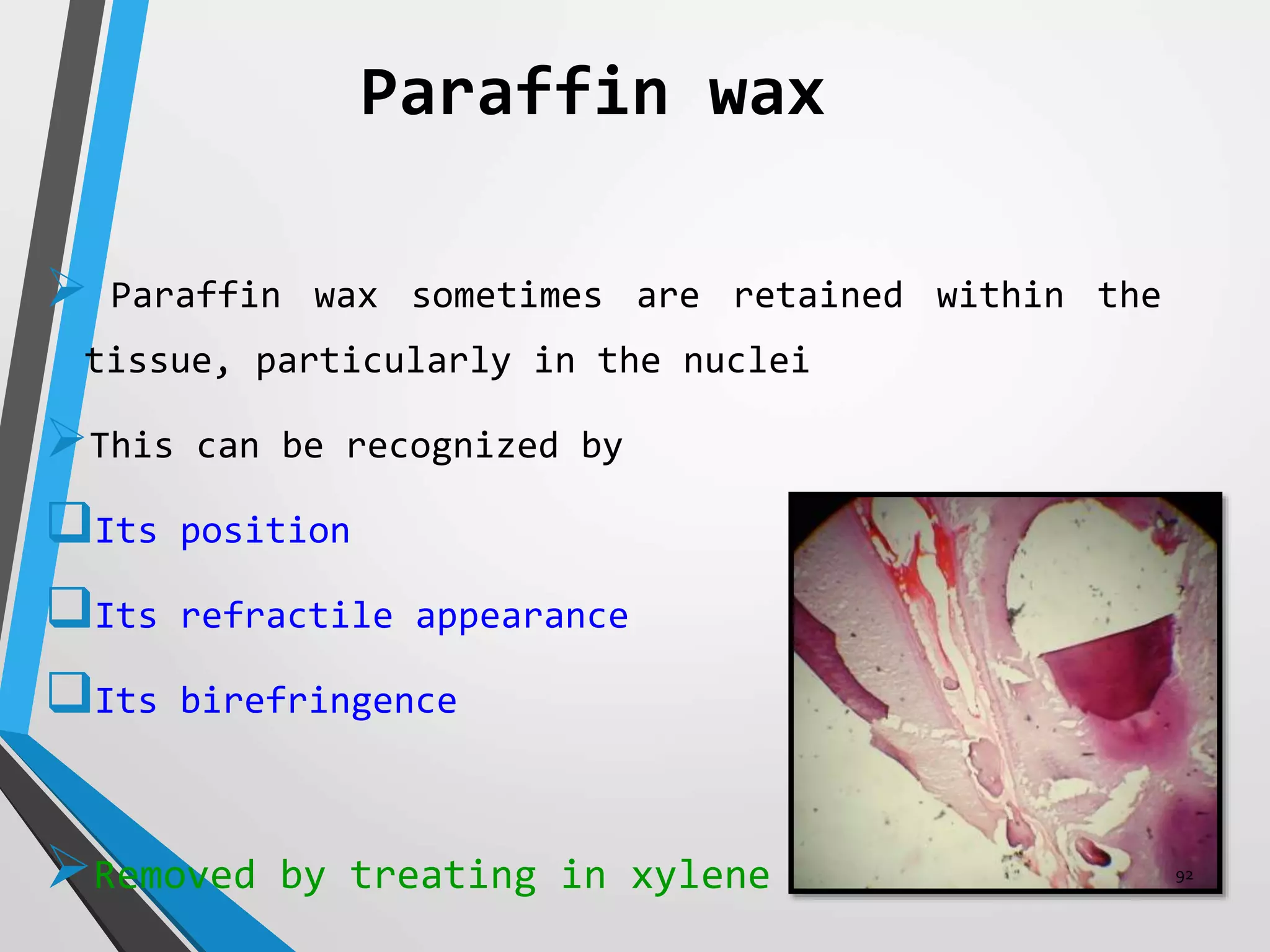
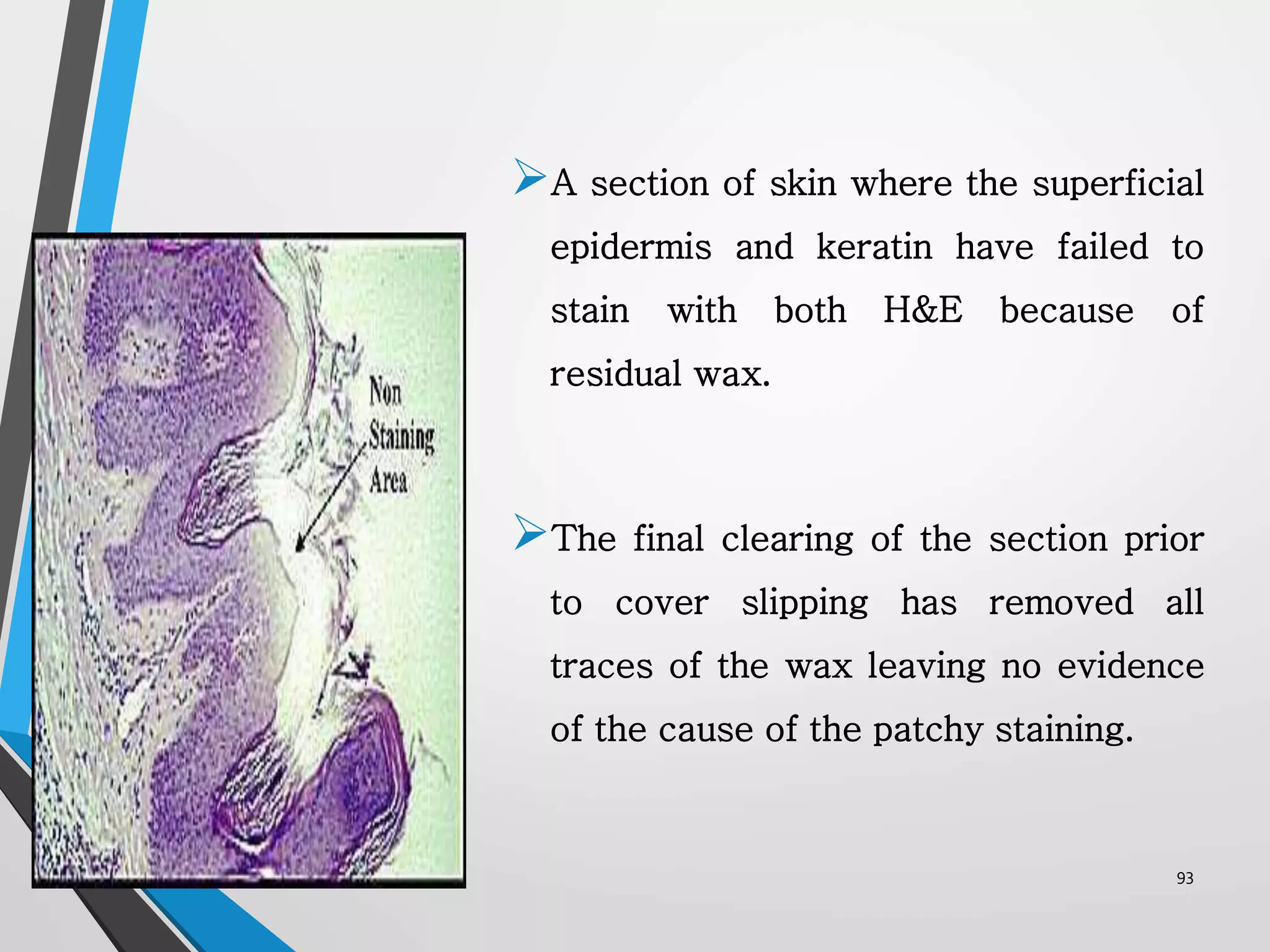
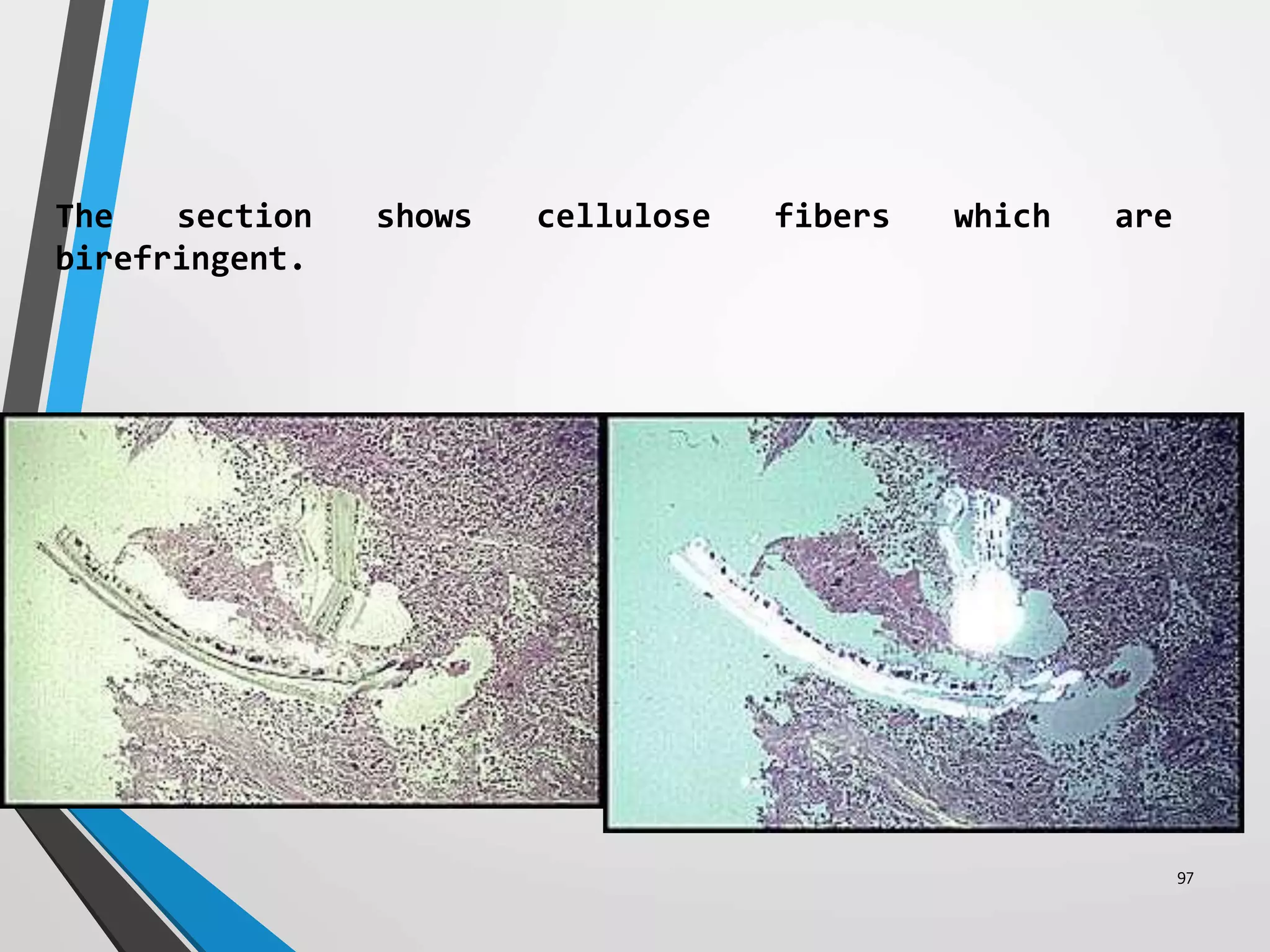
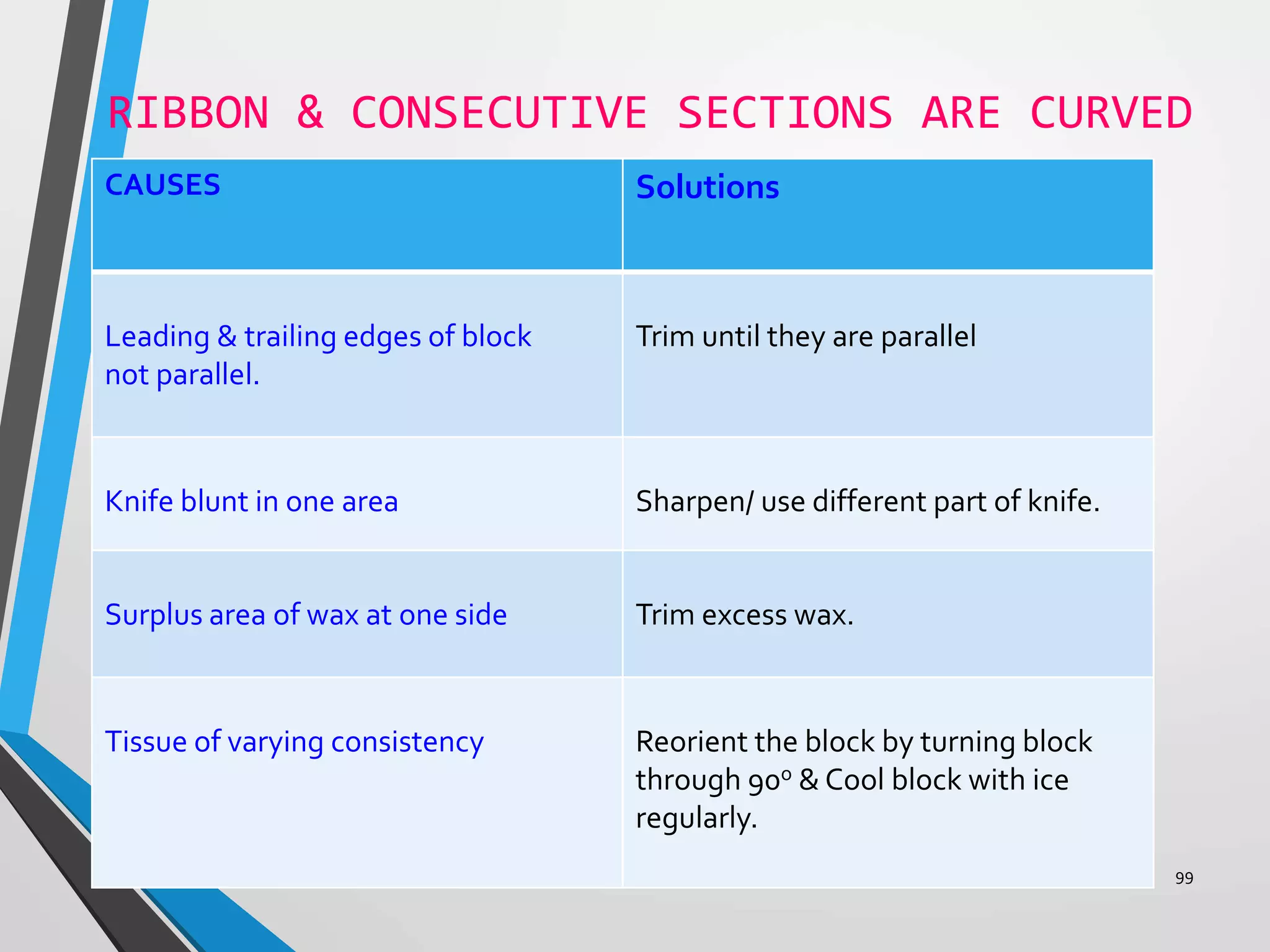
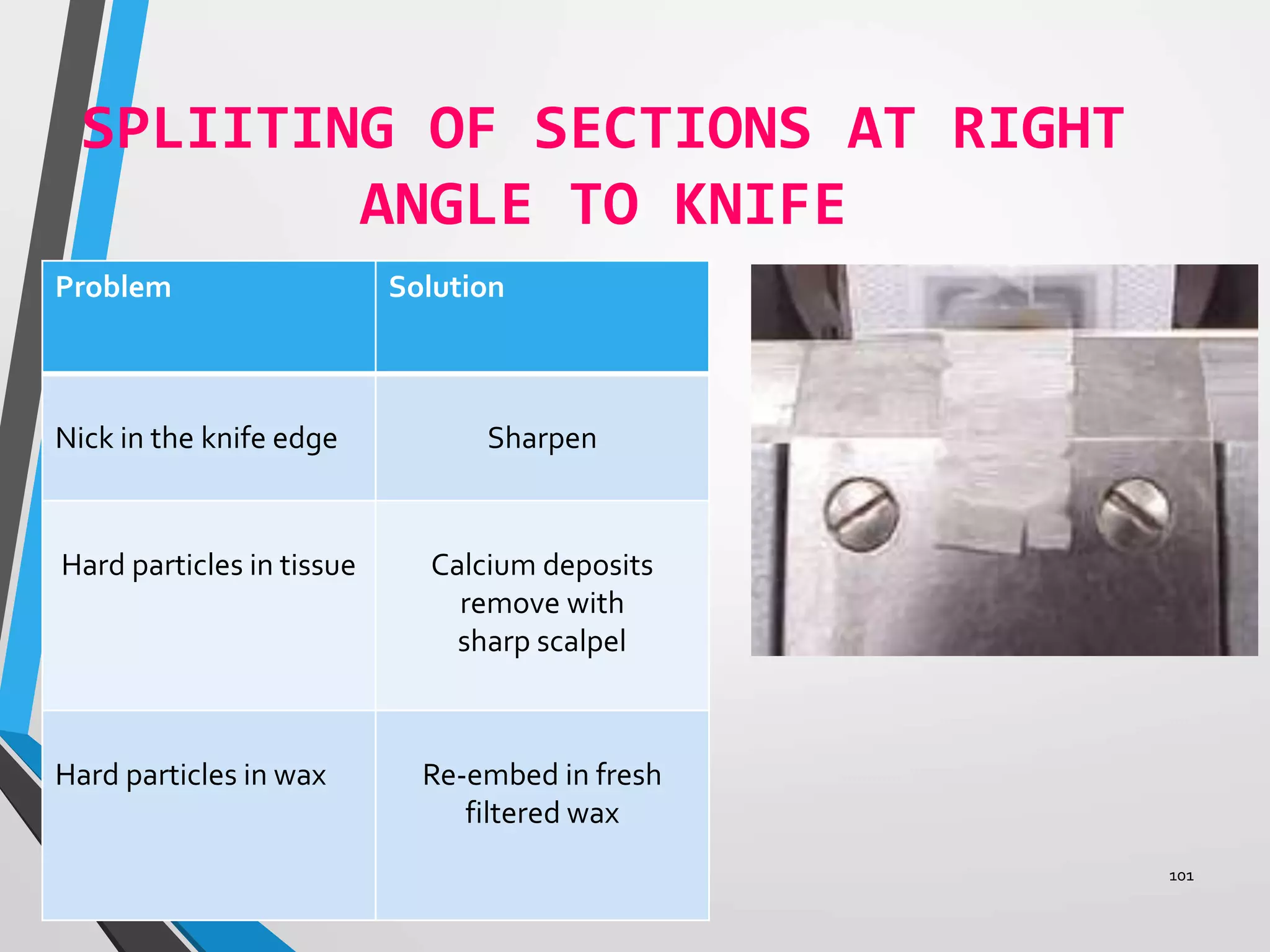
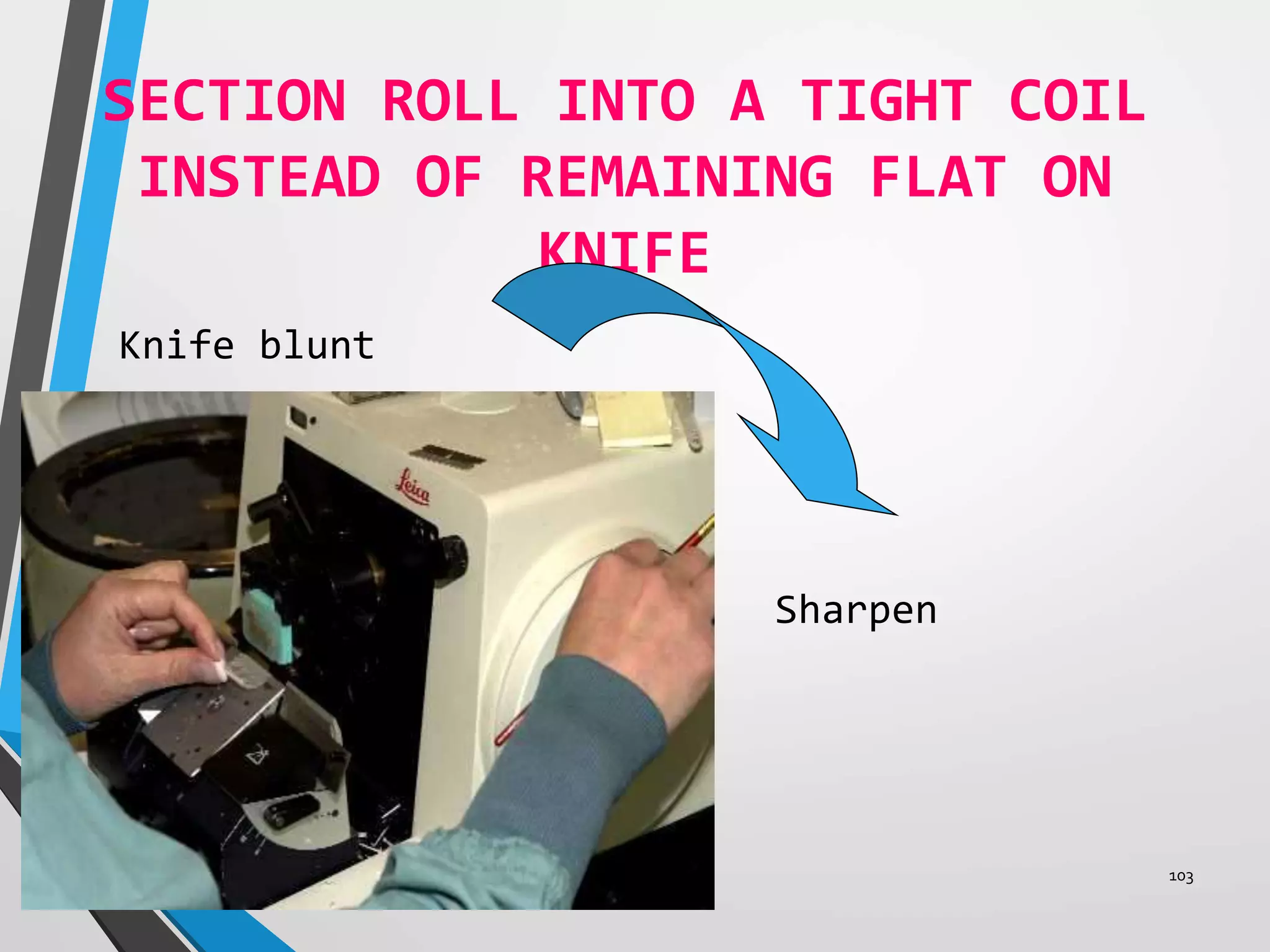
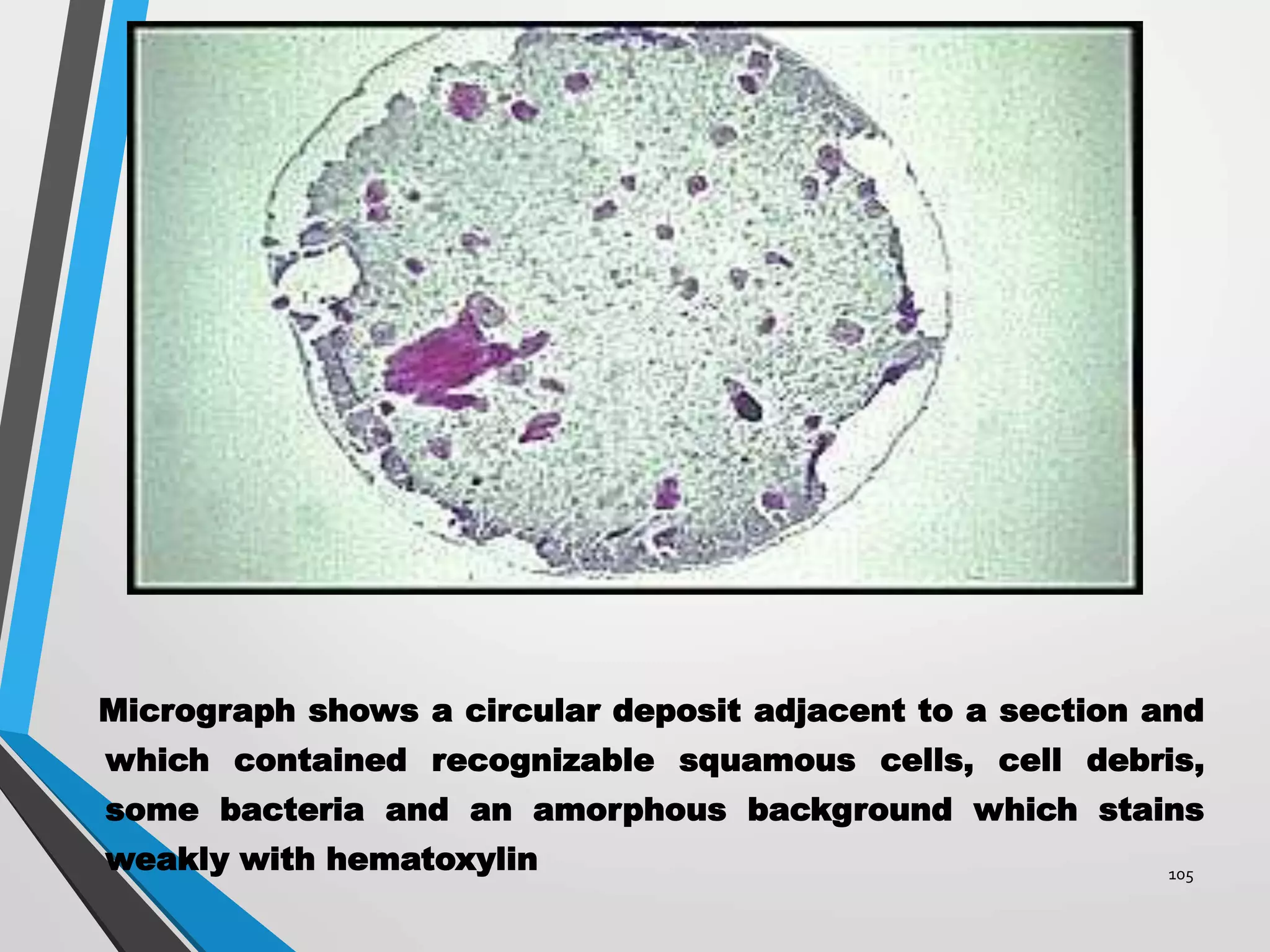
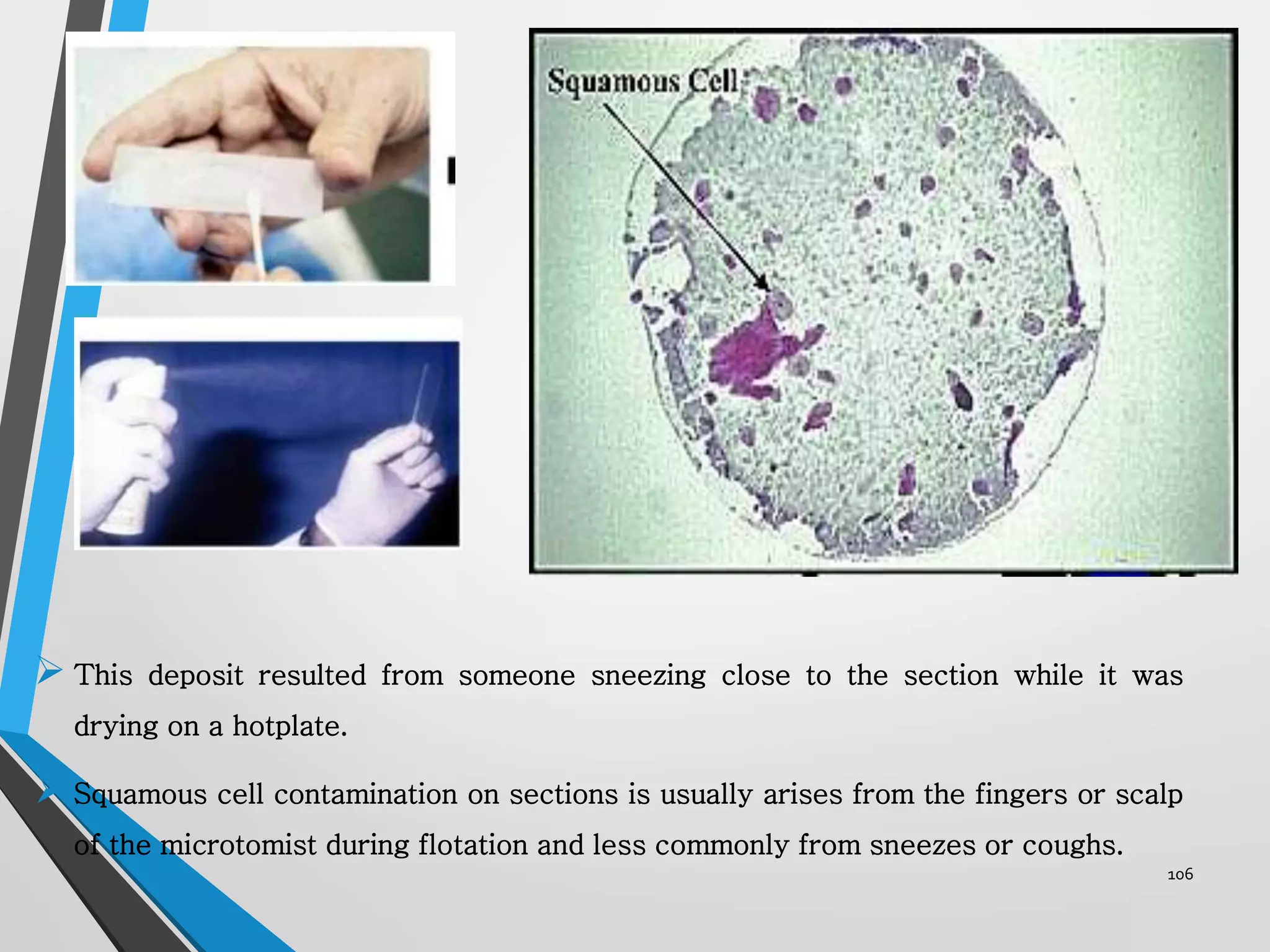
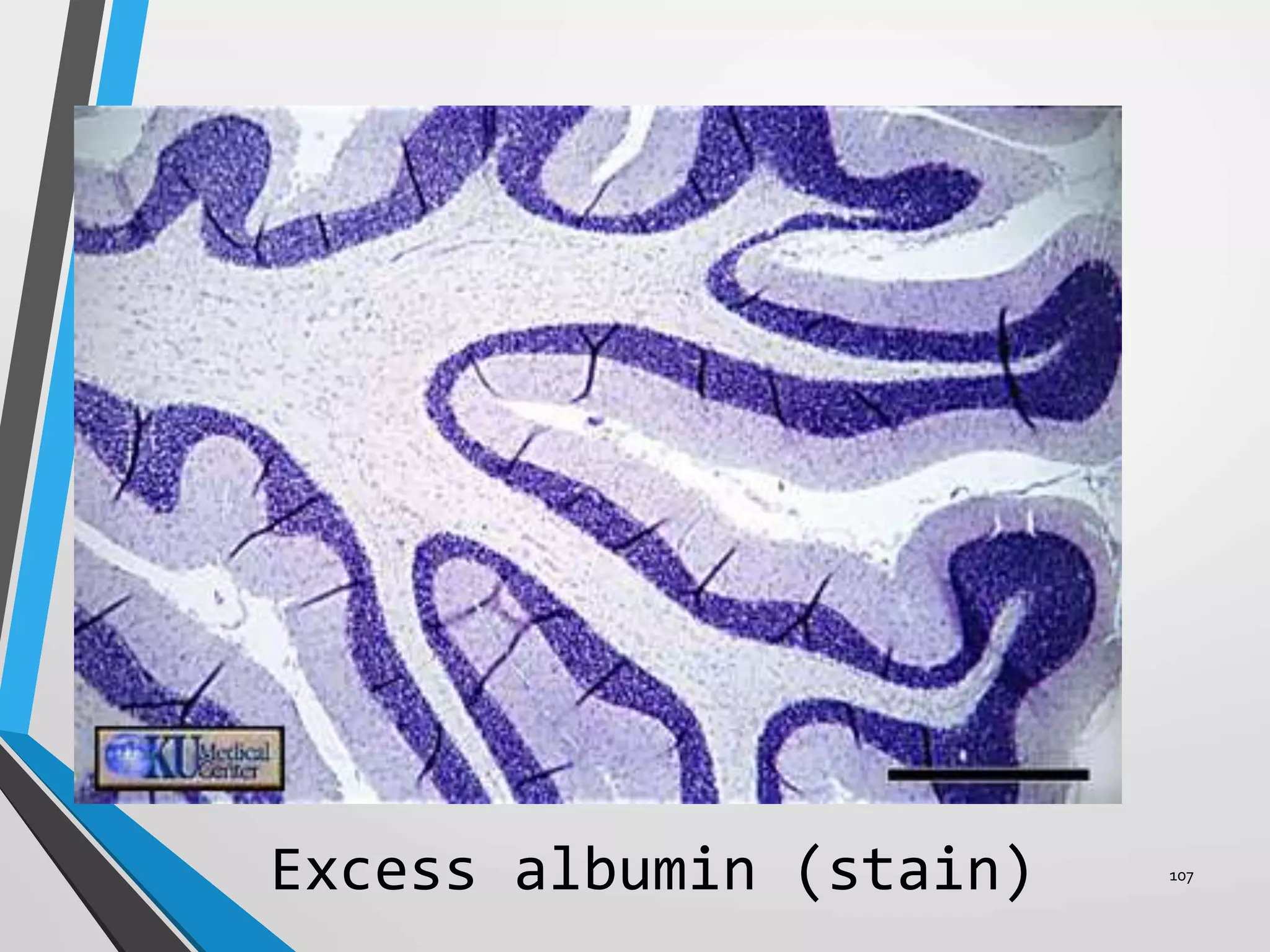
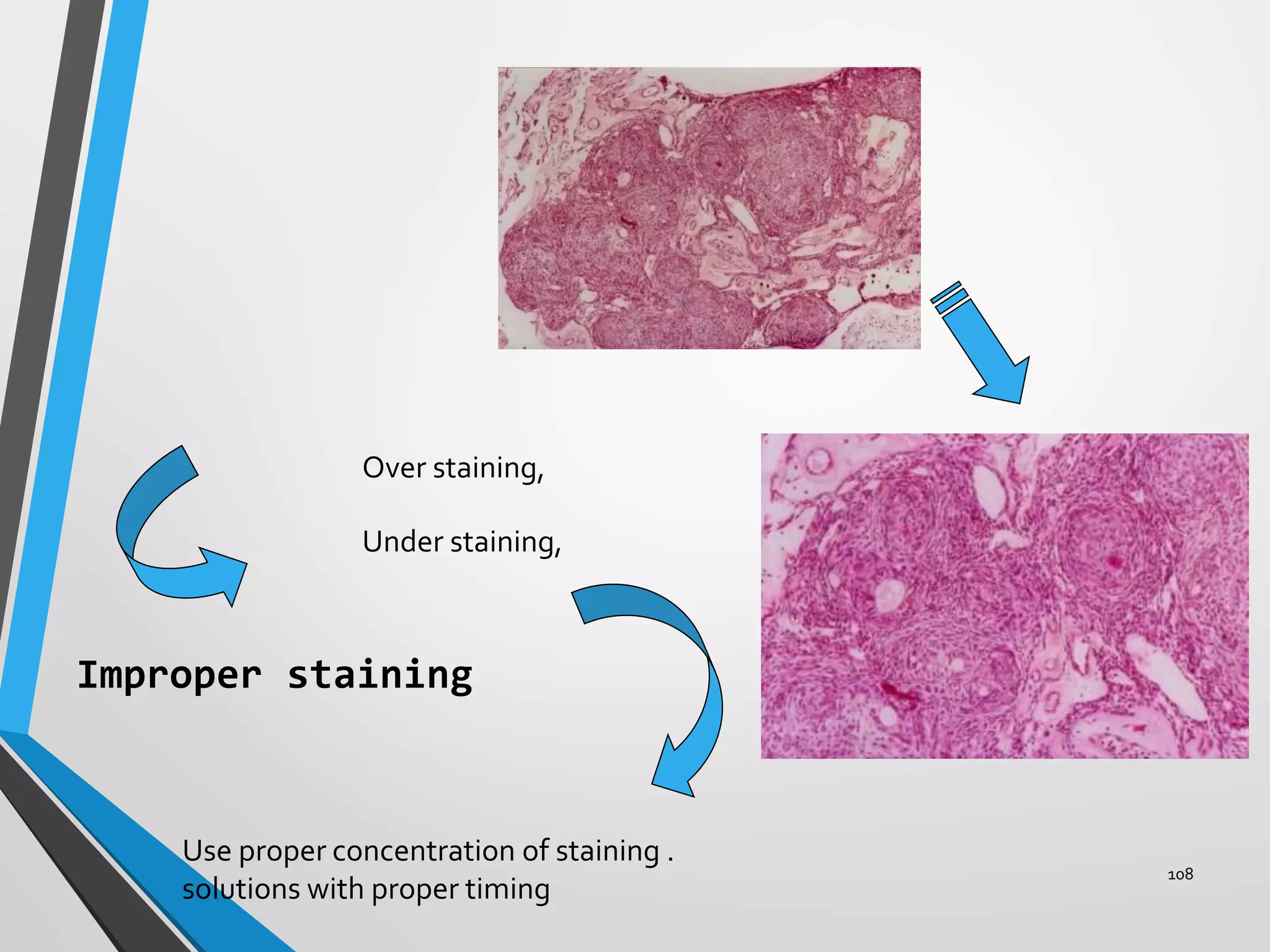
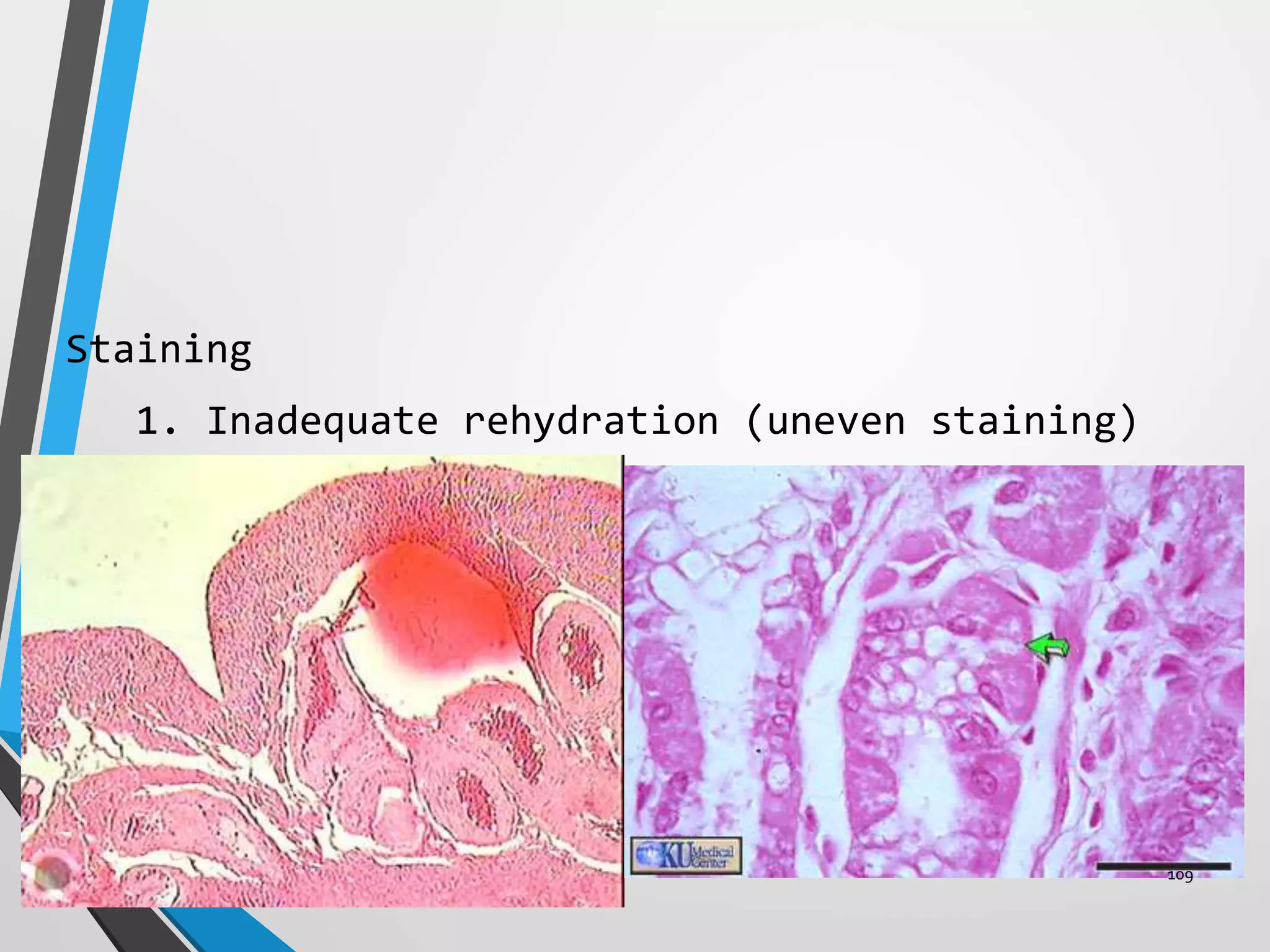
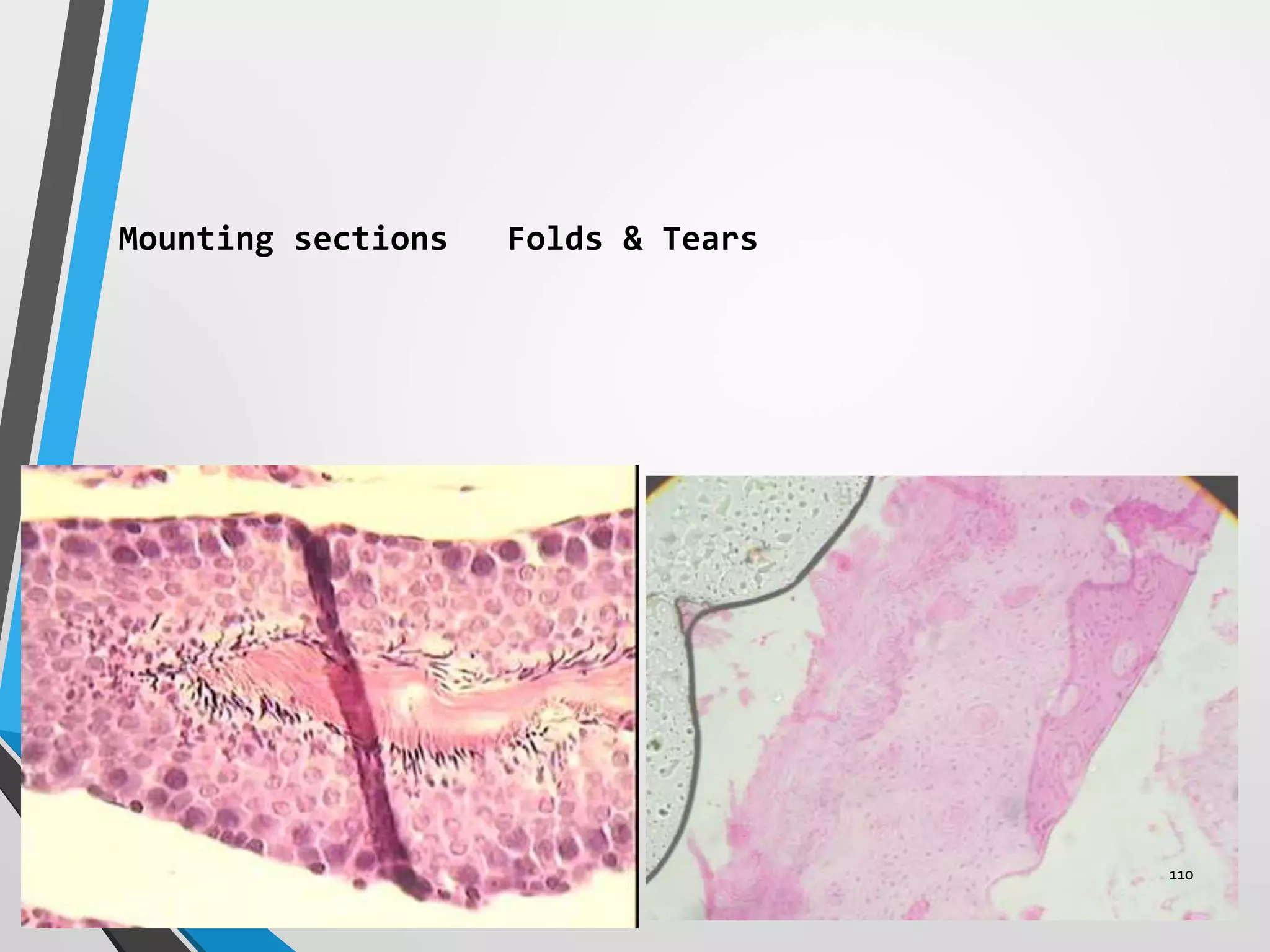
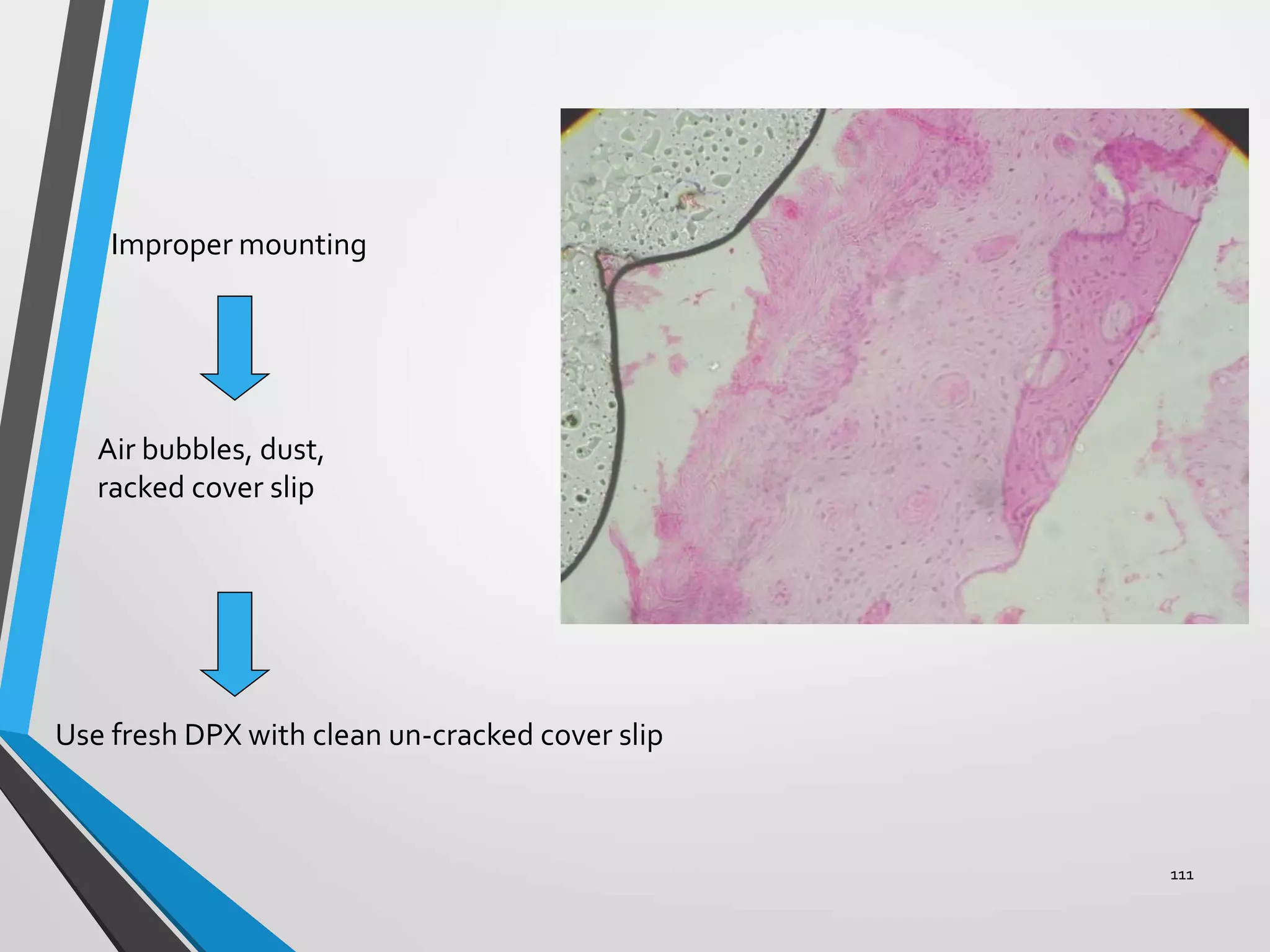
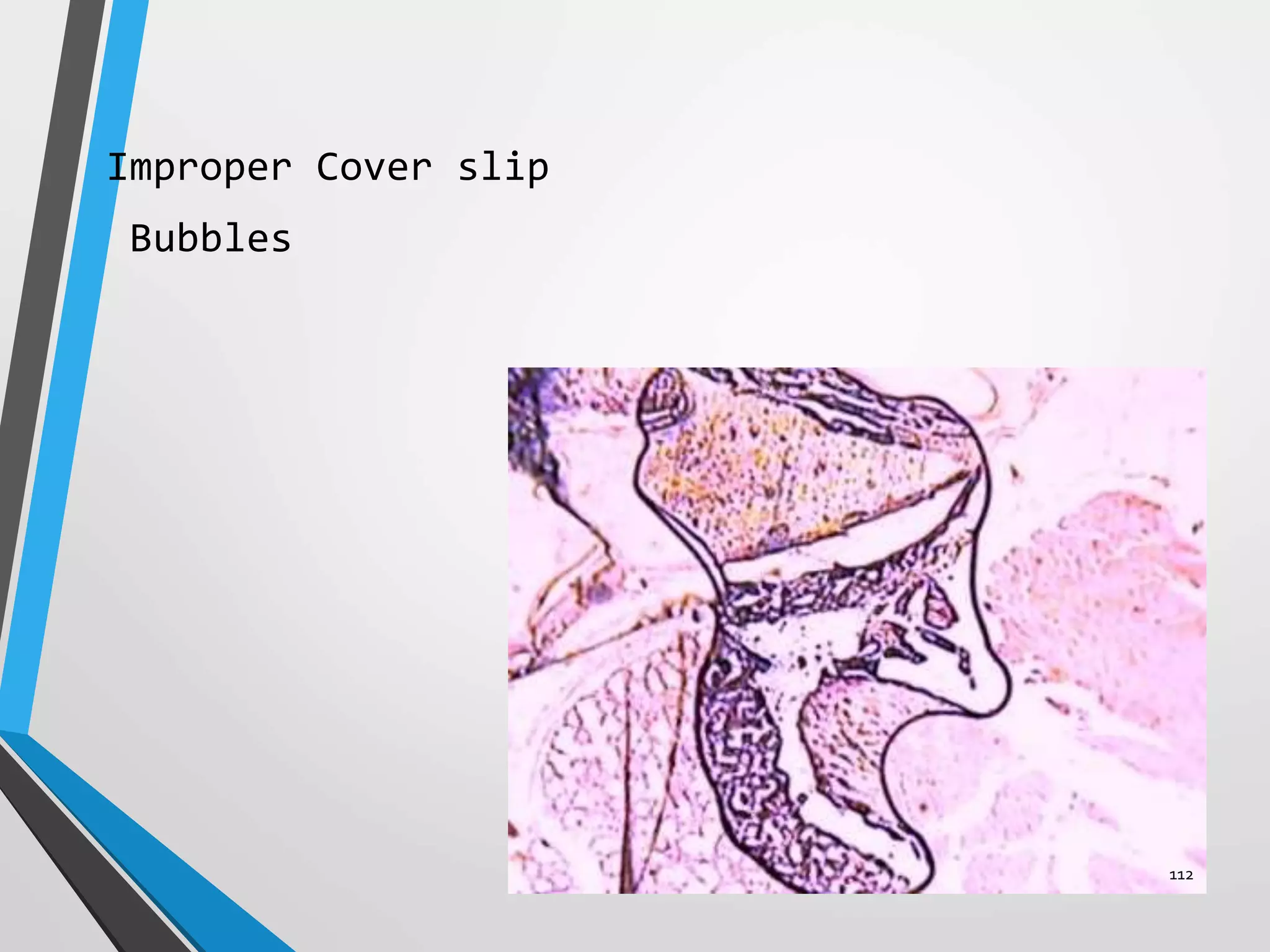
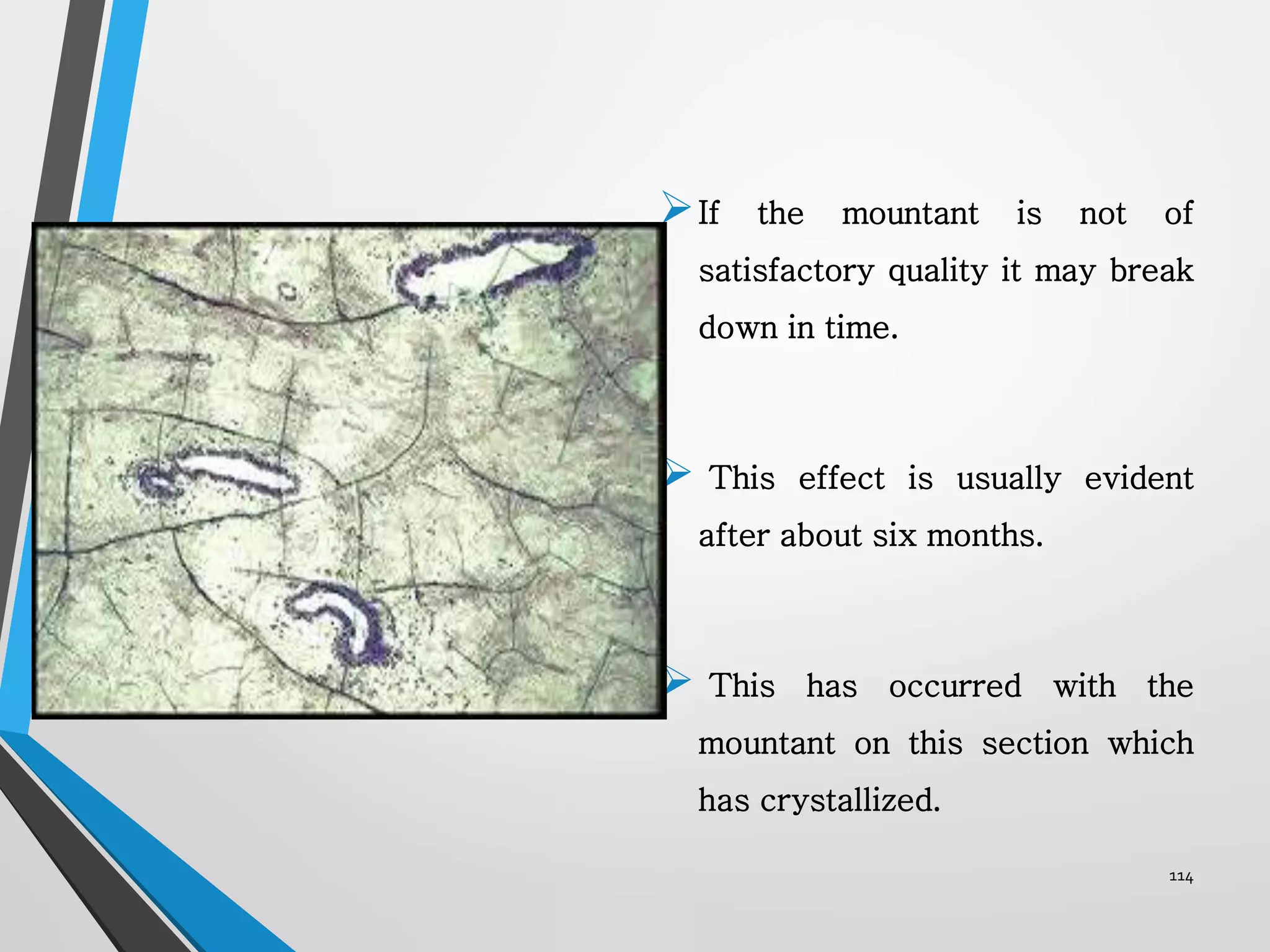
This document discusses various artifacts that can occur during biopsy and tissue processing. It defines an artifact as a defect or distortion resulting from how the tissue was handled from biopsy to fixation. Artifacts can occur during tissue manipulation, transport, fixation, processing, embedding and sectioning. Specific artifacts discussed include squeeze artifacts from improper forceps use, heat artifacts from electrosurgery, autolysis artifacts from delayed fixation, curling artifacts from thin specimens, and orientation artifacts from unlabeled specimens. Proper techniques such as gentle tissue handling, rapid fixation in adequate formalin volume, and specimen orientation labeling can prevent many artifacts.