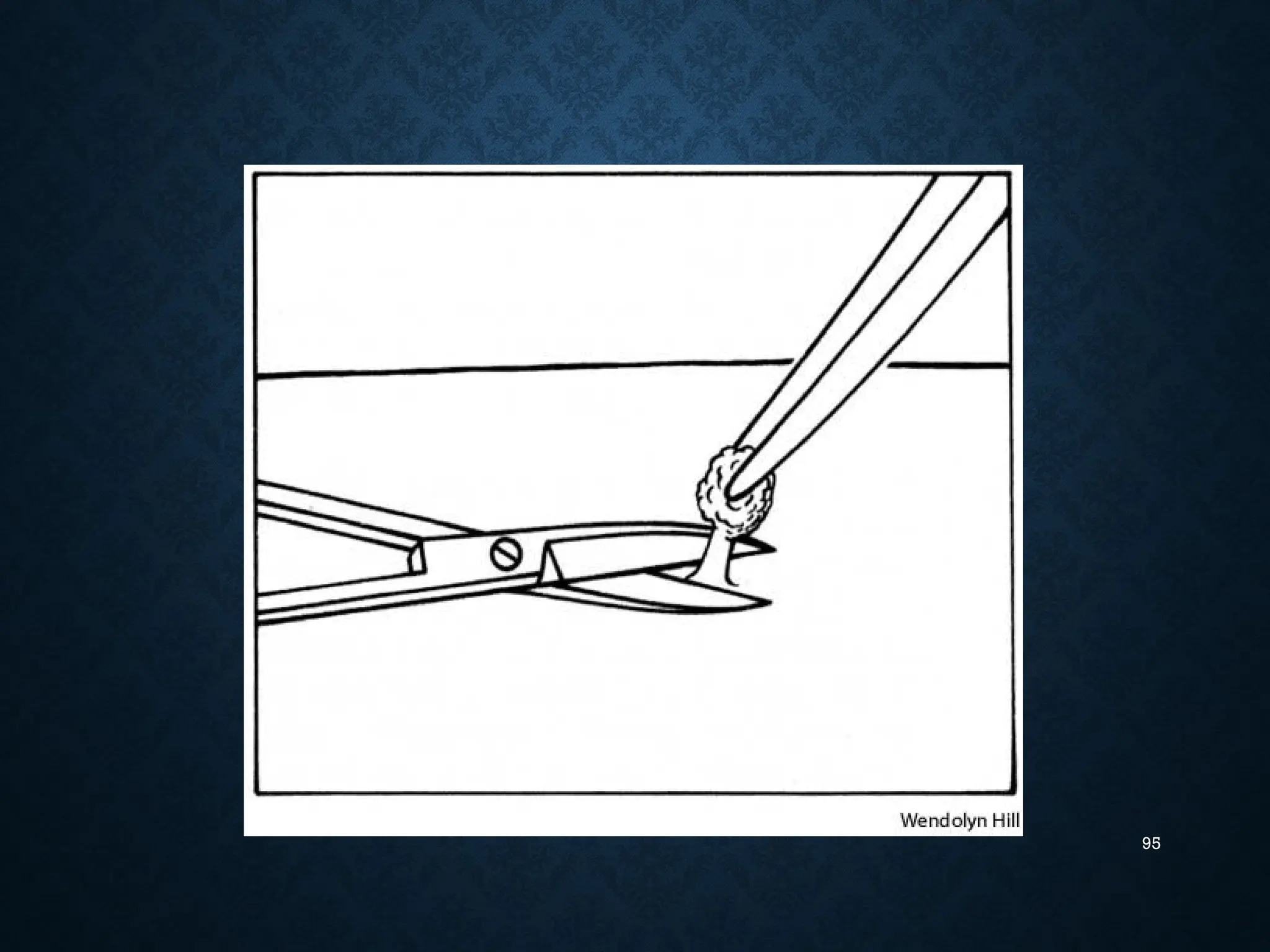
The document provides an in-depth overview of oral biopsy, discussing its historical background, definitions, indications and contraindications, various biopsy techniques, and essential principles. It emphasizes the importance of obtaining a suitable tissue sample for diagnosis while minimizing discomfort for patients. Additionally, it covers specifics about different biopsy methods such as incisional, excisional, punch, and needle biopsies, as well as special considerations related to the procedure.