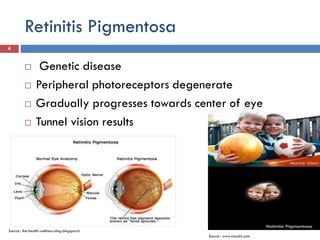
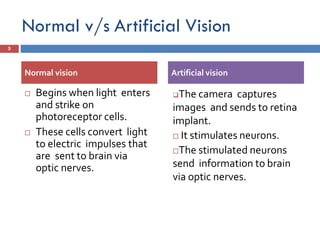
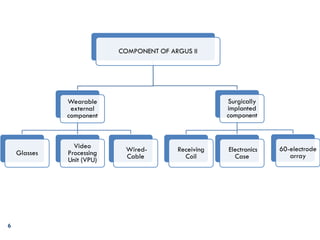
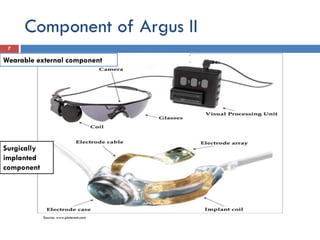
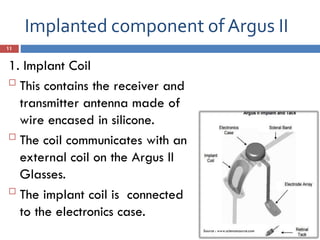
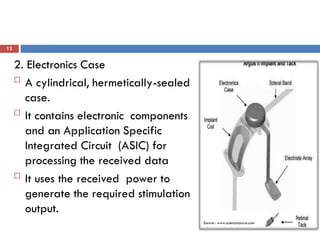
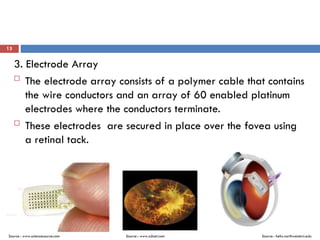
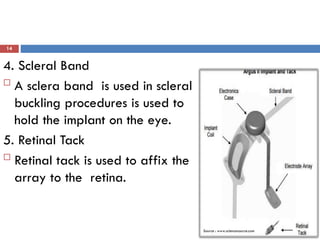
The Argus II retinal prosthesis system is an electronic implant designed to partially restore vision for individuals blinded by retinal degenerative diseases, specifically retinitis pigmentosa. Developed by Second Sight Medical Products, it received CE marking in 2011 and FDA approval in 2013, consisting of external components like glasses and video processing units, along with an implanted electrode array. While it enhances mobility and social engagement for patients, it has limitations including the need for surgical implantation and a high cost of approximately $150,000.