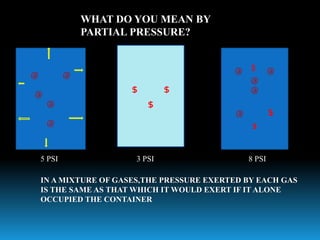
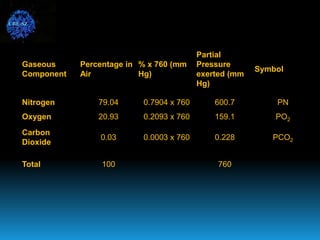
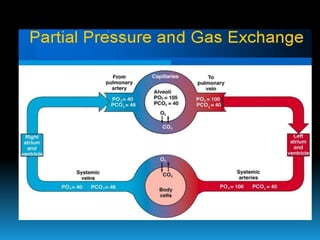
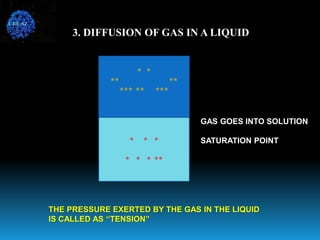
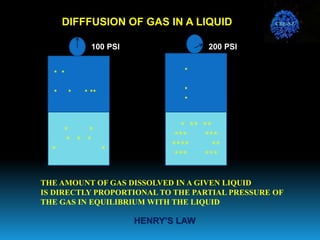
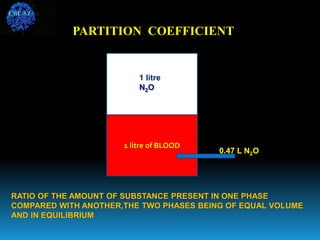
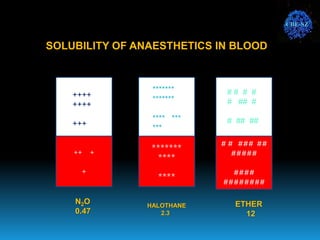
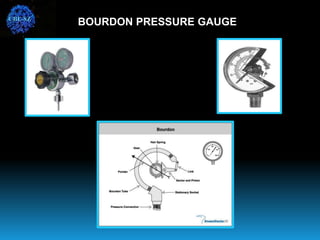
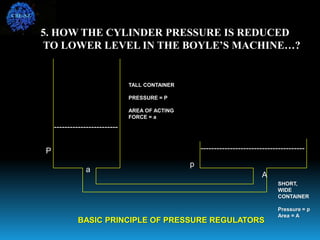
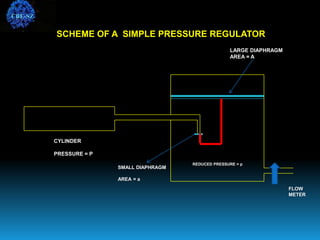
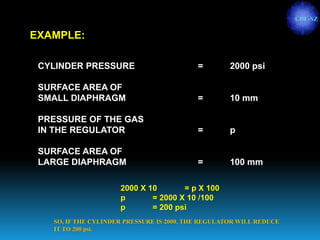
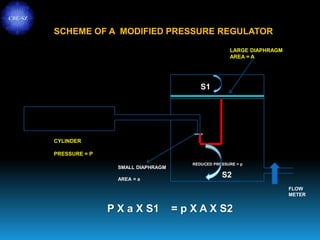
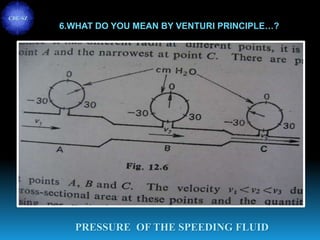
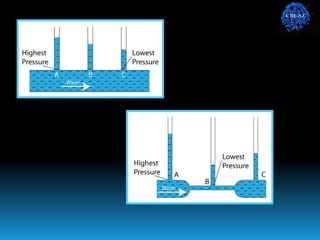
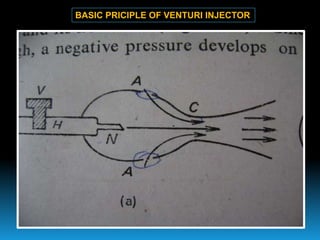
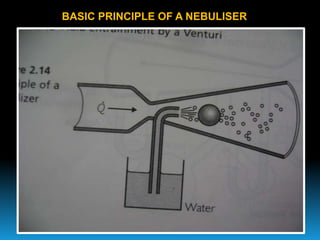
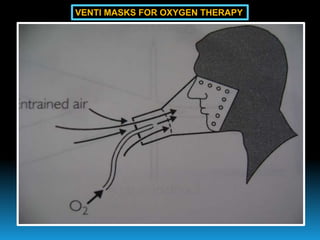
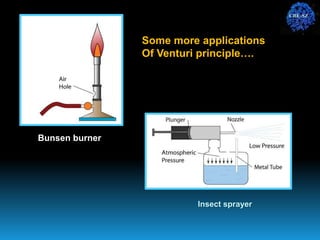
The document discusses the importance of physics in anesthesiology, covering various topics such as the calculation and storage of N2O gas, alveolar gas exchange, and the principles of gas diffusion using Henry's Law. It explains key concepts like partial pressure, Dalton's law, and the function of pressure regulators, alongside practical applications such as the Venturi principle in medical devices. Overall, it highlights how understanding physics is essential for anesthesiologists in their daily practice.