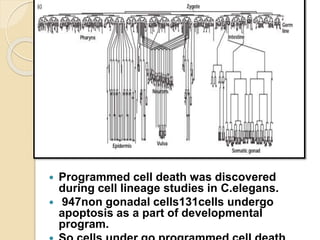
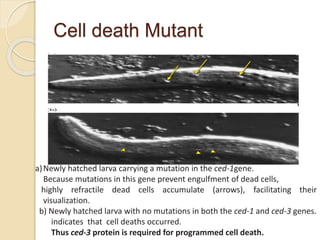
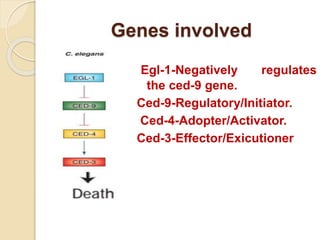
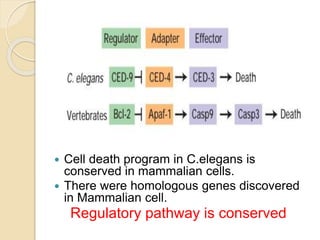
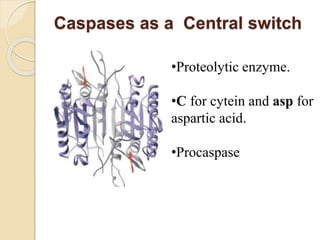
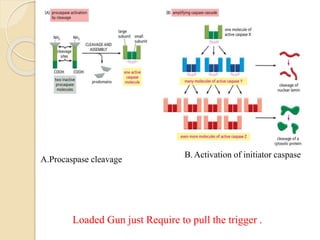
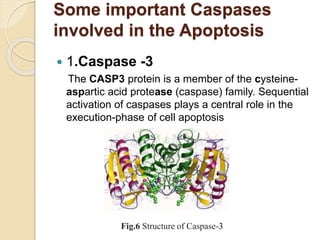
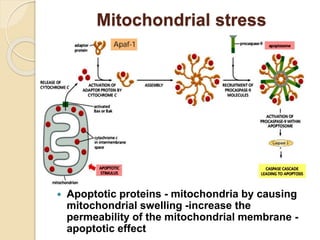
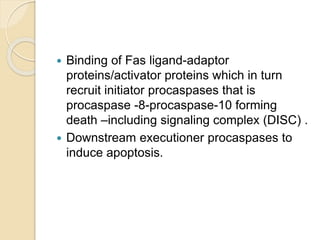
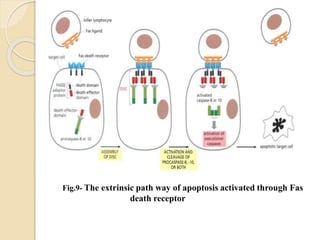
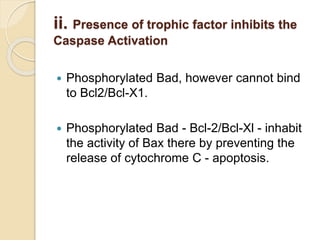
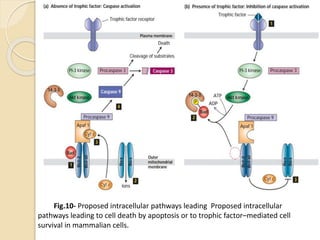
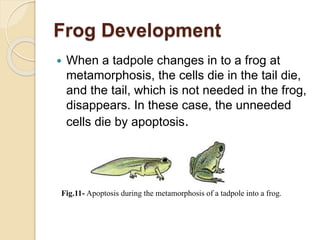
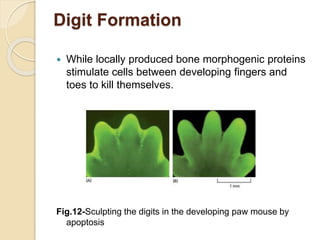
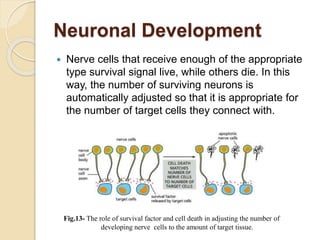
The document discusses the role of caspases as central regulators of apoptosis, detailing their discovery, genetic involvement, and the pathways (intrinsic and extrinsic) that lead to programmed cell death. It emphasizes the importance of apoptosis in normal development and its involvement in diseases such as cancer and neurodegenerative disorders. Key findings include the conservation of cell death pathways across species and the effects of regulatory proteins and signals on apoptosis.