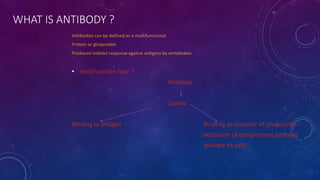
1. Antibodies are Y-shaped proteins called immunoglobulins that are produced by plasma cells in response to antigens like bacteria or viruses.
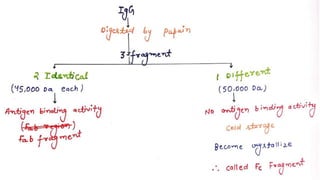
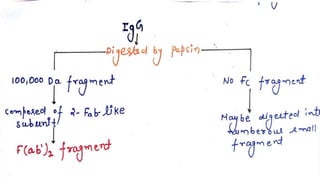
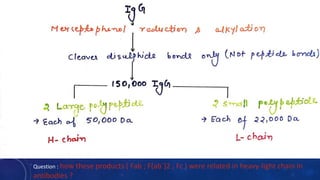
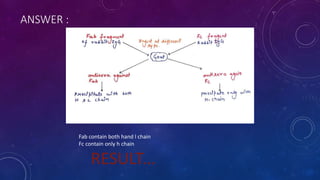
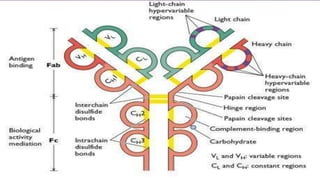
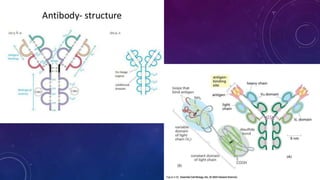
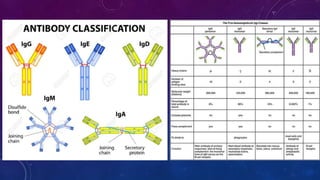
2. Immunoglobulins are composed of light and heavy chains that make up the variable and constant regions, and there are 5 major classes - IgG, IgA, IgM, IgD, and IgE that have different structures and functions.
3. IgG is the most abundant antibody and activates the complement system, IgA protects mucosal surfaces, IgM is the first antibody produced in responses and activates complement, IgD function is not fully known but involved in B cell activation, and IgE triggers allergic reactions by binding mast cells and bas