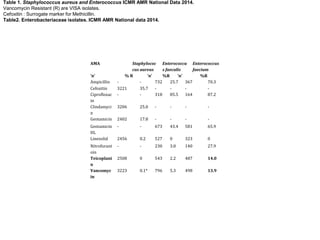
This document contains an antibiogram and antibiotic policy from January to March 2018 from Dr. Pankaj Omar, Chief Intensivist. It includes data on positive and negative culture samples from blood, urine, pus, respiratory and other body sites. The most common organisms identified include Klebsiella, E. coli, Acinetobacter, Pseudomonas, Candida, Staphylococcus. The document also provides principles of empirical antimicrobial therapy for sepsis and septic shock in ICU patients and lists common multidrug resistant organisms and appropriate treatment options.
















































![represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more.
In patients admitted from the community or emergency department, it can be assumed that patients had no pre-existing organ
dysfunction, baseline SOFA score assumed to be zero. Organ dysfunction can be identified in these patients by the quick SOFA or
qSOFA. The presence of any two of respiratory rate ≥22, altered mentation or systolic blood pressure ≤100 mm Hg identified high risk
of patients. qSOFA is an extremely useful screening tool for organ dysfunction, especially in patients outside the ICU. It can be used to
suspect sepsis and initiate further investigations and treatment.
Septic shock should be defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities
are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by a
vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater and serum lactate level greater than 2 mmol/L
(>18 mg/dL) in the absence of hypovolemia](https://image.slidesharecdn.com/antibiogram-180417135113/85/Antibiogram-50-320.jpg)