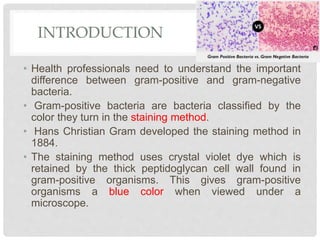
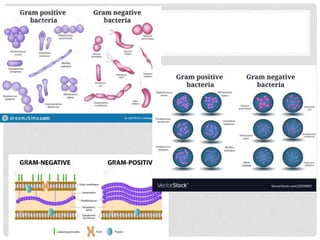
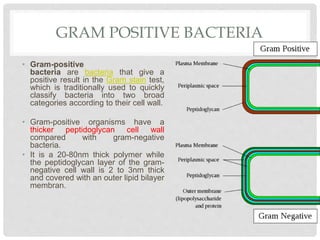
This document discusses gram positive sepsis and toxic shock syndrome. It begins by differentiating between gram positive and gram negative bacteria, noting that gram positive bacteria have a thicker peptidoglycan cell wall. It then discusses epidemiology of gram positive infections, evaluation and management of suspected sepsis, initial resuscitative therapy including IV fluids and antibiotics, monitoring response to therapy, identifying infection sources, and managing patients who fail or respond to initial therapy. It concludes by describing toxic shock syndrome caused by Staphylococcus aureus.

![EPIDEMIOLOGY
• Bloodstream infection mortality rates have increased by
78% in just two decades[1].
• Gram-positive organisms have a highly variable growth
and resistance patterns.
• The SCOPE project (Surveillance and Control of
Pathogens of Epidemiologic Importance) found that
gram-positive organisms in those with an underlying
malignancy accounted for 62% of all bloodstream
infections in 1995 and for 76% in 2000 while gram-
negative organisms accounted for 22% and 14% of
infections for these year.](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-6-320.jpg)







![• Choosing a regimen — The choice of antimicrobials
can be complex and should consider the patient's history
(eg, recent antibiotics received, previous organisms),
comorbidities (eg, diabetes, organ failures), immune
defects (eg, human immune deficiency virus), clinical
context (eg, community- or hospital-acquired), suspected
site of infection, presence of invasive devices, Gram
stain data, and local prevalence and resistance patterns
[46-50].
• antimicrobial choice should be tailored to each
individual.](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-17-320.jpg)

![• Methicillin-resistant S. aureus – There is growing recognition
that methicillin-resistant S. aureus (MRSA) is a cause of
sepsis not only in hospitalized patients, but also in community
dwelling individuals without recent hospitalization [52,53]. For
these reasons, we suggest empiric
intravenous vancomycin (adjusted for renal function) be
added to empiric regimens, particularly in those with shock or
those at risk for MRSA. Potential alternative agents to
vancomycin (eg, daptomycin for non-pulmonary
MRSA, linezolid) should be considered for patients with
refractory or virulent MRSA, or with a contraindication to
vancomycin](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-19-320.jpg)



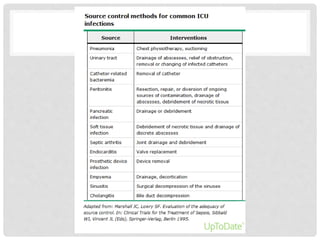




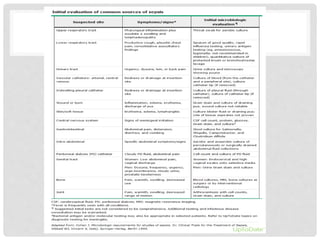
![DE-ESCALATION FLUIDS
• Patients who respond to therapy (ie, clinical hemodynamic
and laboratory targets are met; usually hours to days) should
have the rate of fluid administration reduced or stopped,
vasopressor support weaned, and, if necessary, diuretics
administered.
• While early fluid therapy is appropriate in sepsis, fluids may
be unhelpful or harmful when the circulation is no longer fluid
responsive.
• Careful and frequent monitoring is essential because patients
with sepsis may develop cardiogenic and noncardiogenic
pulmonary edema (ie, acute respiratory distress syndrome
[ARDS]).](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-29-320.jpg)








![DIAGNOSIS
• The diagnosis of staphylococcal TSS is established based on
clinical and laboratory criteria
• Detection of S. aureus in culture is not required for the diagnosis of
staphylococcal TSS.
• S. aureus is recovered from blood cultures in approximately 5
percent of cases [24]; it is recovered from wound or mucosal sites in
80 to 90 percent of cases [62].
• According to the United States Centers for Disease Control and
Prevention (CDC), a confirmed case is a case that meets the
following clinical criteria: fever, hypotension, diffuse erythroderma,
desquamation (unless the patient dies before desquamation can
occur), and involvement of at least three organ systems, with
cultures negative for alternative pathogens and serologic tests
negative for other conditions (if obtained). A patient who is missing
one of the above clinical criteria may be considered a probable
case.](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-40-320.jpg)


![ANTIBIOTIC THERAPY
• Empiric therapy — For empiric treatment of sepsis of unknown cause that
might represent staphylococcal TSS, we favor the following regimen (pending
culture results):
• ●Vancomycin (adults: 15 to 20 mg/kg/dose intravenously [IV] every 8 to 12
hours, not to exceed 2 g per dose; children: 60 mg/kg per day IV in four divided
doses)
• PLUS
• ●Clindamycin (adults: 900 mg IV every eight hours; children: 25 to 40 mg/kg IV
per day in three divided doses)
• PLUS one of the following:
• ●A combination drug containing a penicillin plus beta-lactamase inhibitor
(adults: piperacillin-tazobactam 4.5 g IV every six hours; children
300 mg/kg/day IV in four divided doses)
• ●A carbapenem (adults: imipenem 500 mg IV every six hours or meropenem 1 g
IV every eight hours; children: imipenem 15 to 25 mg/kg/dose every 6 hours
[maximum 4 g per day] or meropenem 25 mg/kg/dose every 8 hours)](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-43-320.jpg)



![• In one case report of a 39-year-old man with HIV
infection and diffuse erythema of the arms and legs,
desquamation (of the arms, hands, feet, and eyebrows),
pharyngeal erythema, and a lesion that grew a TSST-1-
producing S. aureus, IVIG (200 mg/kg per day) was
administered for five days after failing antibiotic therapy;
symptoms subsequently resolved [63].
• ●In a retrospective study of patients with necrotizing
fasciitis and shock associated with group
A Streptococcus or S. aureus, adjunctive IVIG has no
apparent impact in mortality.](https://image.slidesharecdn.com/grampositivesepsis-200221084925/85/Gram-positive-sepsis-47-320.jpg)