This document provides information about the transition element titanium. It discusses the discovery of titanium, its sources in nature, physical and chemical properties, reactions, and common uses. Key points include that titanium is the ninth most abundant element in the Earth's crust, is commonly found in minerals like rutile and ilmenite, and reacts with oxygen, halogens, and acids. It is used to make aircraft and ships due to its high strength and corrosion resistance.




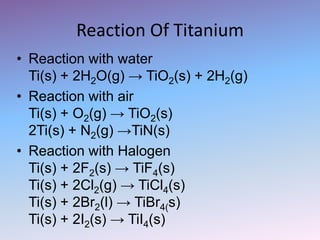
![Atomic number 22
Atomic symbol Ti
Atomic weight 47.88
Electron configuration [Ar]4s23d2
Atomic radius 187 pm
Elektronegativity 1.54
Density 4.54 g/cm3
Melting point 1668 C
Boiling Point 3287 C
Oxidation States 4,3,2
Ionization potential 4.82 V
Crystal stucture Hexagonal](https://image.slidesharecdn.com/anor-131002031152-phpapp02/85/Anor-5-320.jpg)




![Atomic number 40
Atomic symbol Zr
Atomic weight 91.22
Electron configuration [Kr]5s24d2
Atomic radius 186 pm
Elektronegativitas 1.33
Kerapatan 6.506 g/cm3
Melting point 1855 C
Boiling Point 4409 C
Oxidation States 4
Ionization potential 6.84 V
Crystal stucture Hexagonal](https://image.slidesharecdn.com/anor-131002031152-phpapp02/85/Anor-11-320.jpg)



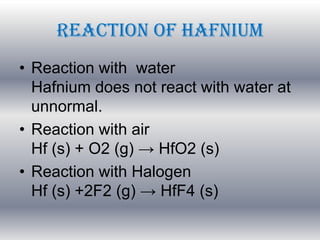
![Atomic number 72
Atomic symbol Hf
Atomic weight 178.49
Electron configuration [Xe]6s24f145d2
Atomic radius 212 pm
Elektronegativitas 1.30
Density 13,31 g/cm3
Melting point 2233 C
Boiling Point 4602 C
Oxidation States 4
Ionization potential 6.65
Crystal stucture Hexagonal](https://image.slidesharecdn.com/anor-131002031152-phpapp02/85/Anor-15-320.jpg)