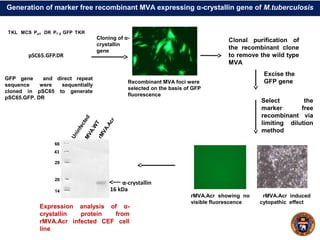
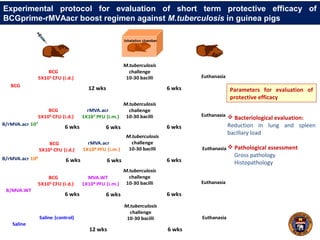
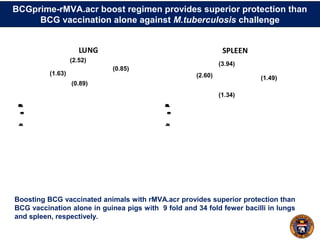
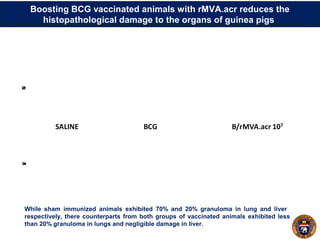
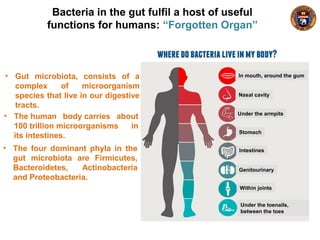
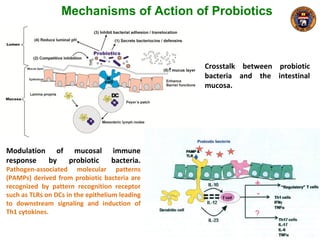
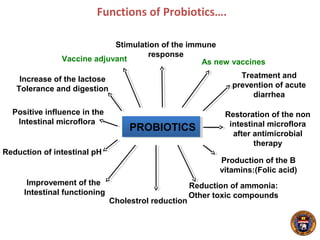
This document summarizes research on using a "BCG prime - DNA boost" vaccination strategy for tuberculosis. Key points:
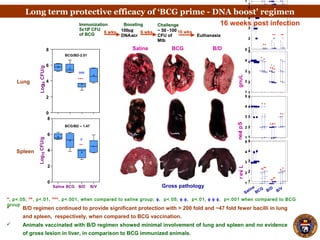
- Mice vaccinated with BCG followed by a boost with DNA encoding the M. tuberculosis antigen α-crystallin had significantly reduced lung and spleen bacterial loads compared to BCG alone after airborne infection.
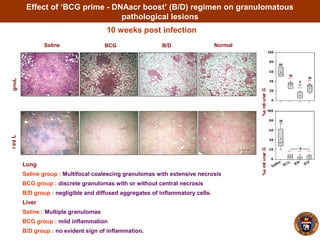
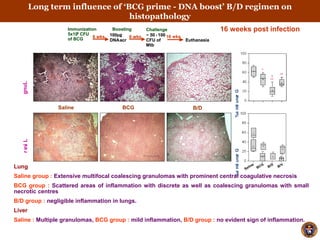
- The boosted mice also had less severe lung, liver and spleen pathology and granulomas.
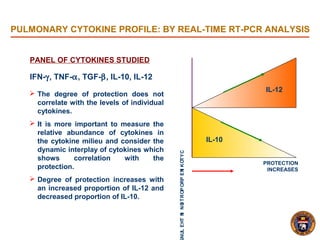
- Protection lasted for at least 16 weeks and was associated with an increased proportion of the cytokines IL-12 and decreased IL-10 in the lungs.
- The results suggest boosting BCG with α-crystallin DNA enhances and prolongs protection against tuberculosis







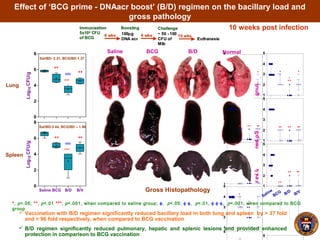
![B/D vaccination confers enhanced protection against M. tuberculosis challenge in mice. The
figure depicts the bacillary load in lungs and spleen of mice at 4 weeks post-infection.
Vaccine induced protection was associated with increased frequency of PPD and antigen
specific multifunctional CD4 T cells (2+ and 3+) along with higher production of IFN-γ, TNF-
α and IL-2.
Induction of CD4 Th1 cell responses by BCG-DNAacr (B/D) regimen
Frequency of CD4 T cells producing different cytokines (1+
, 2+
and 3+
) along with MFI
and iMFI for these cytokines are compared in spleen among the vaccinated groups at 12
weeks post-immunization
[B]1.69
0.67
1.84
1.35](https://image.slidesharecdn.com/anilk-150413102648-conversion-gate01/85/Anil-k-tyagi-20-320.jpg)