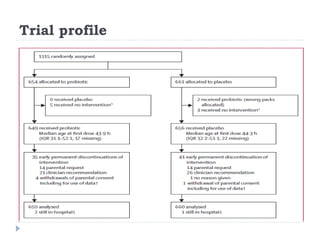
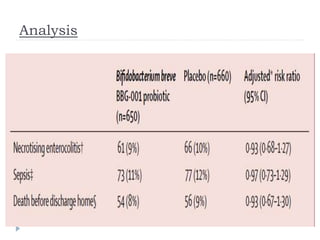
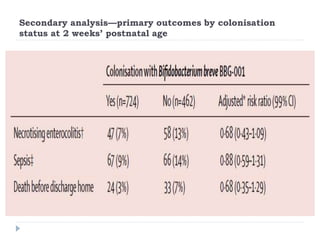
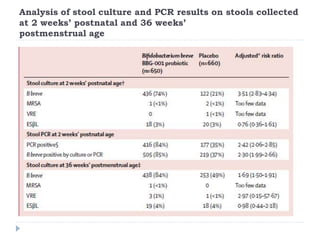
This randomized controlled trial tested the effectiveness of the probiotic Bifidobacterium breve BBG-001 in reducing necrotizing enterocolitis, late-onset sepsis, and death in very preterm infants. The trial recruited 1315 infants from 24 hospitals in the UK who were randomly assigned to receive either the probiotic or placebo within 48 hours of birth. The results showed no evidence of benefit for the probiotic intervention in reducing the three primary outcomes of necrotizing enterocolitis, sepsis over 72 hours, or death before hospital discharge. This large, well-designed trial does not support the routine use of this probiotic for very preterm infants.