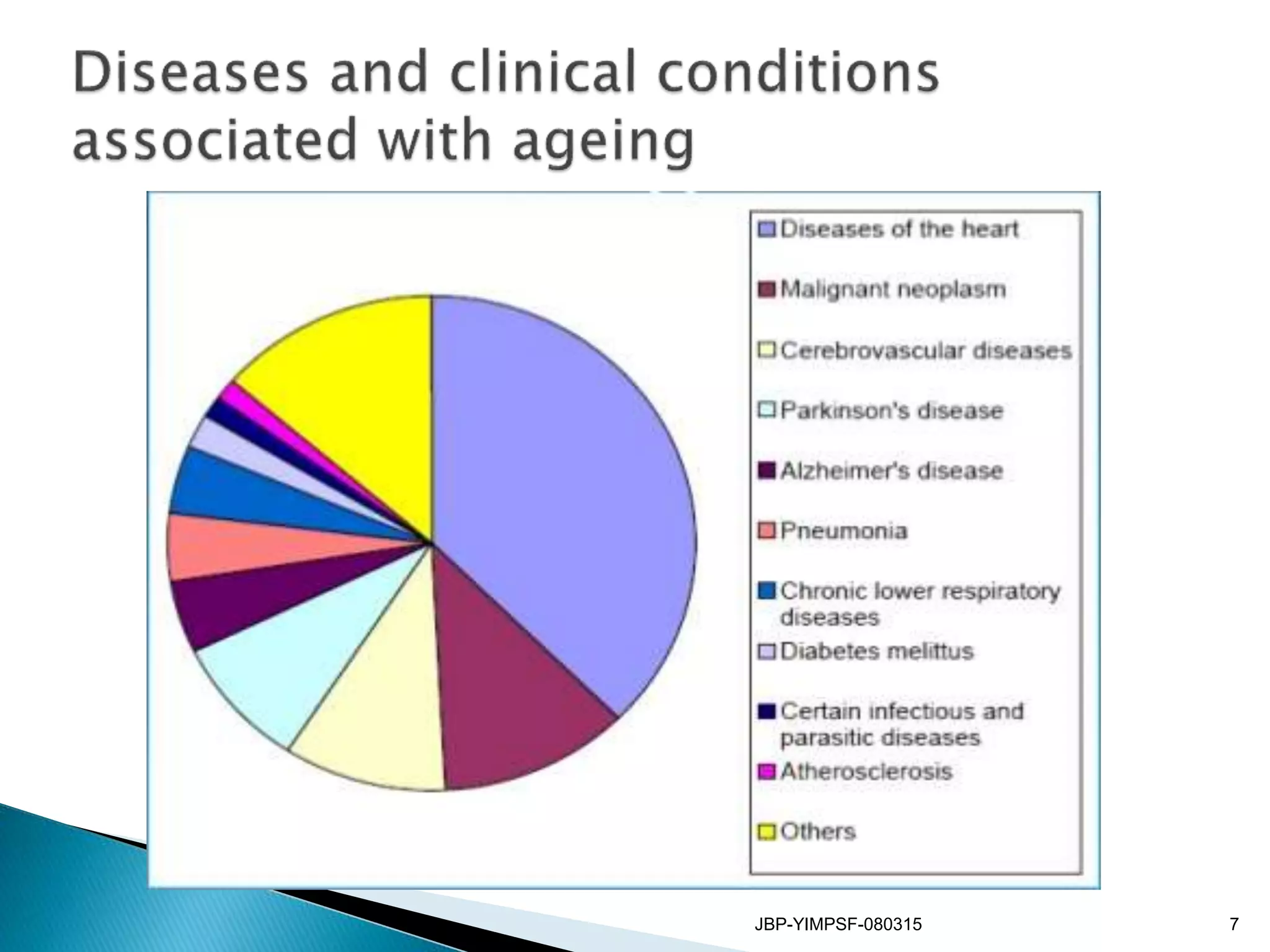
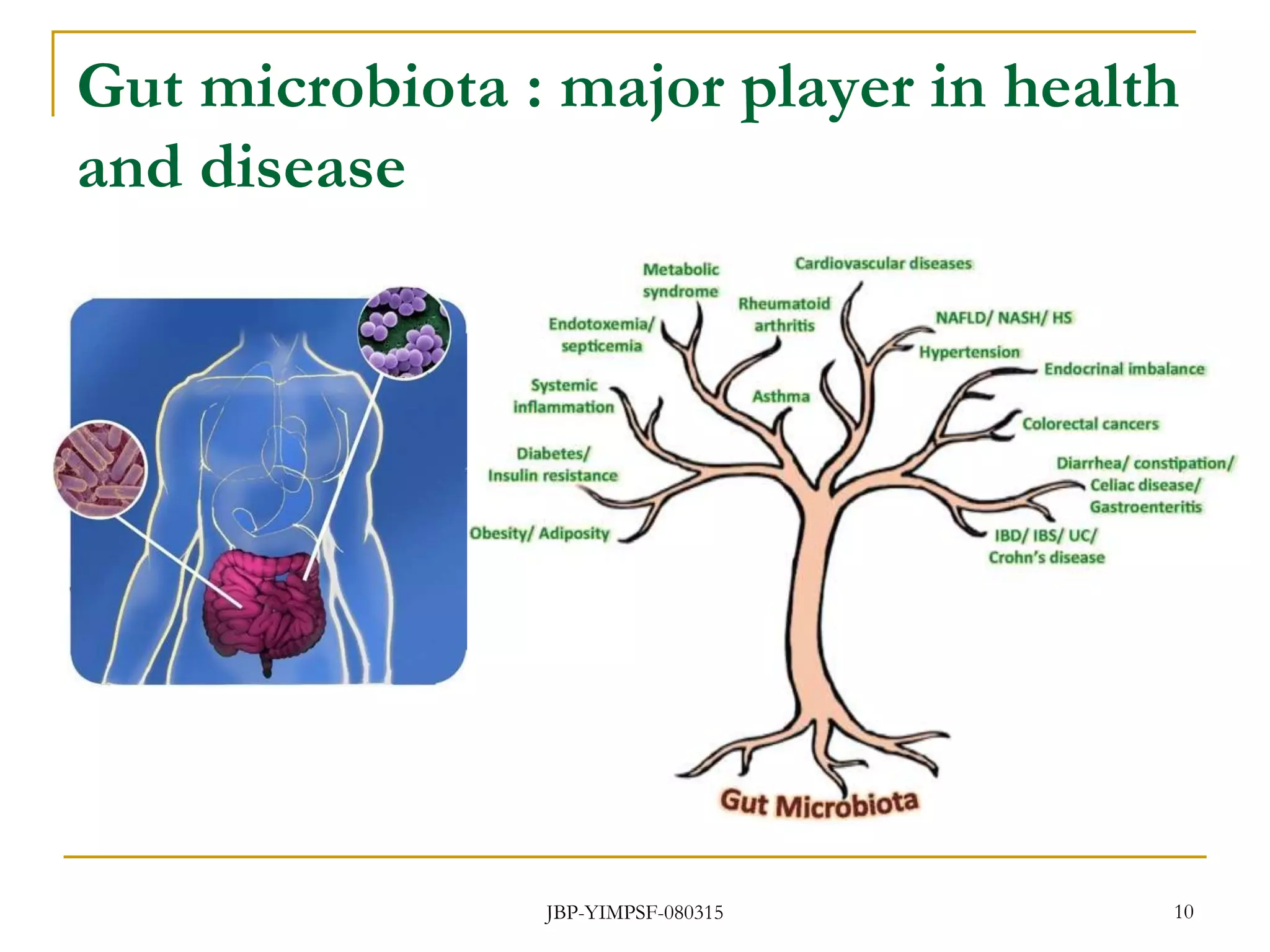
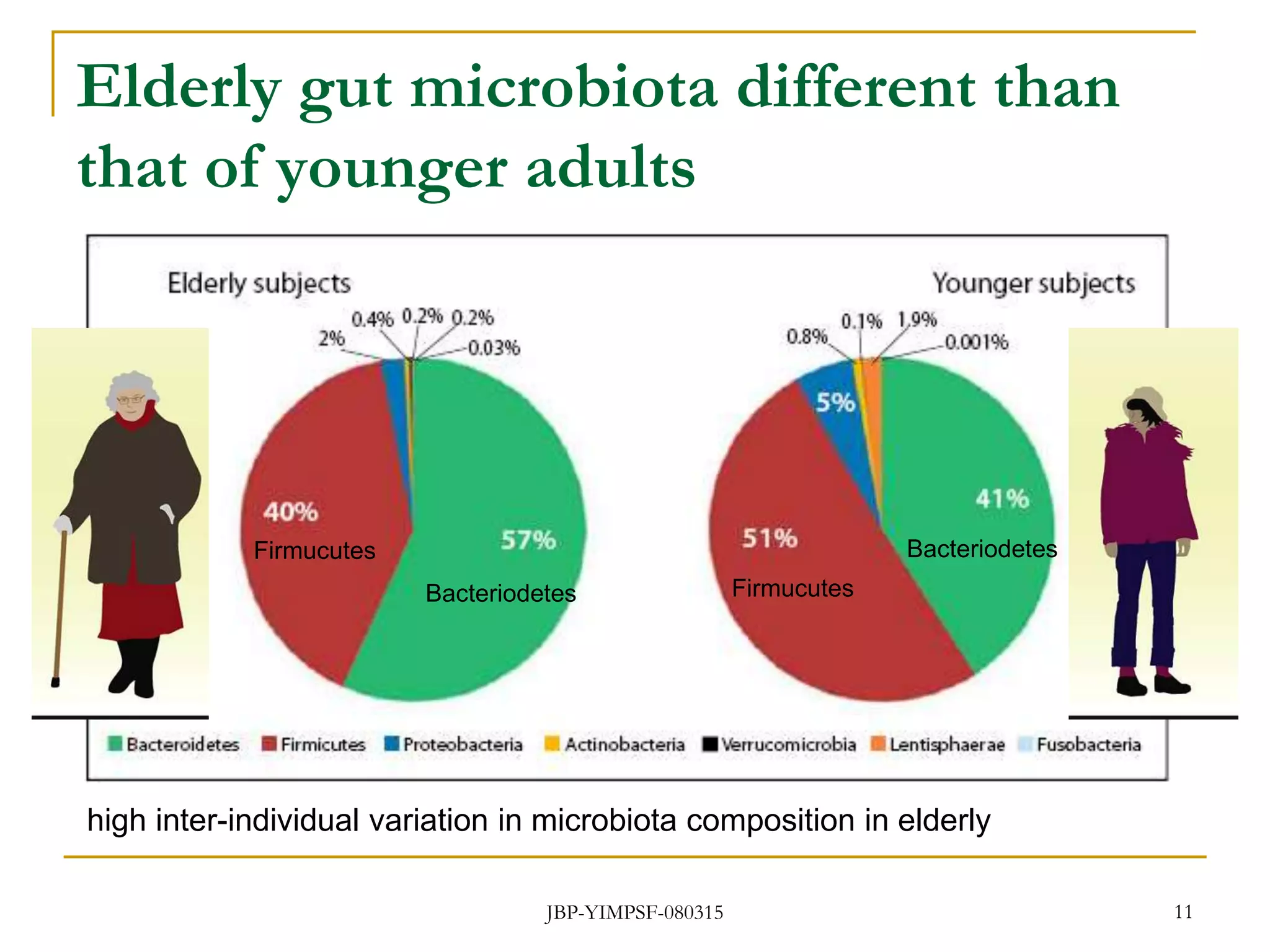
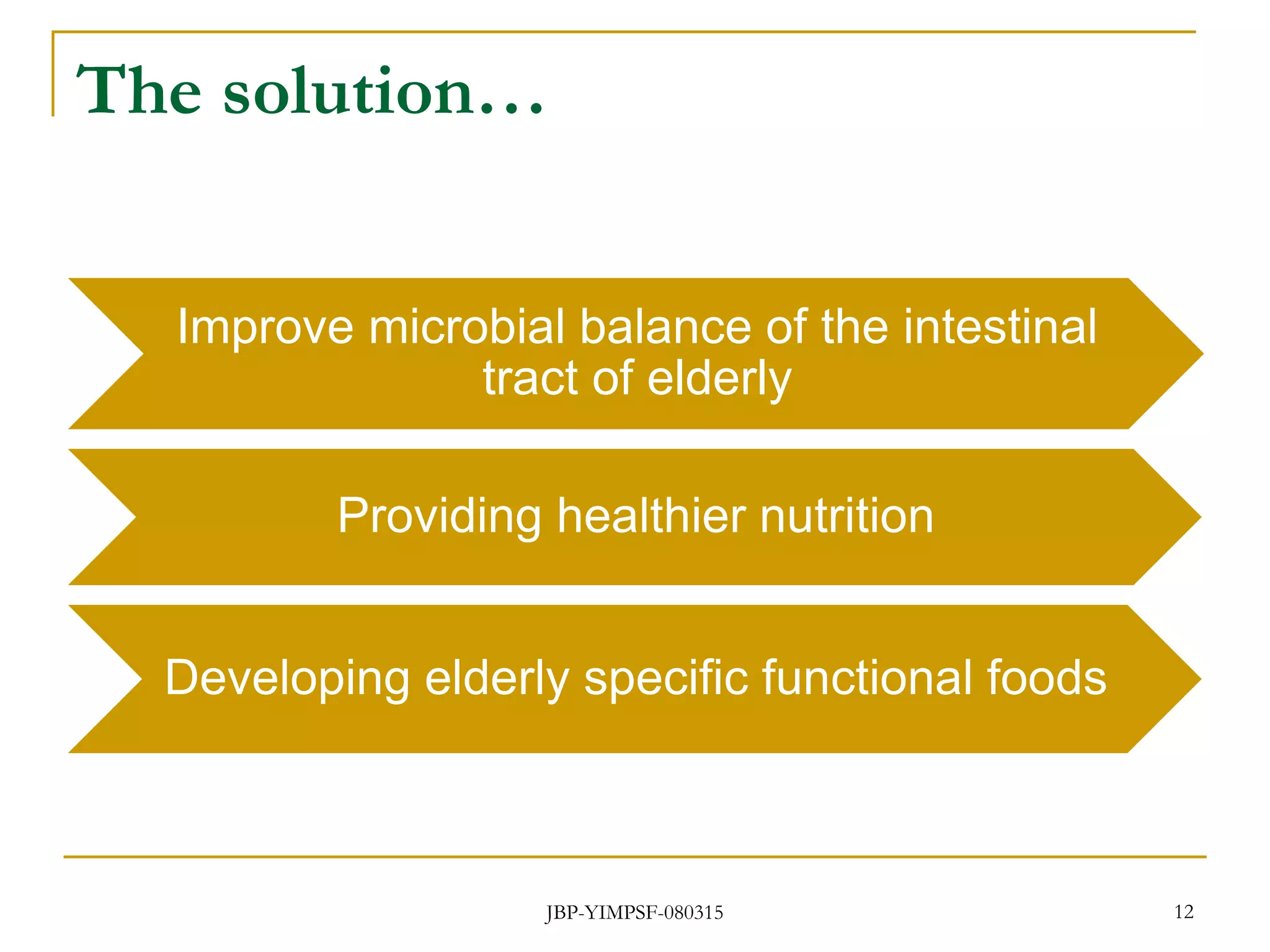
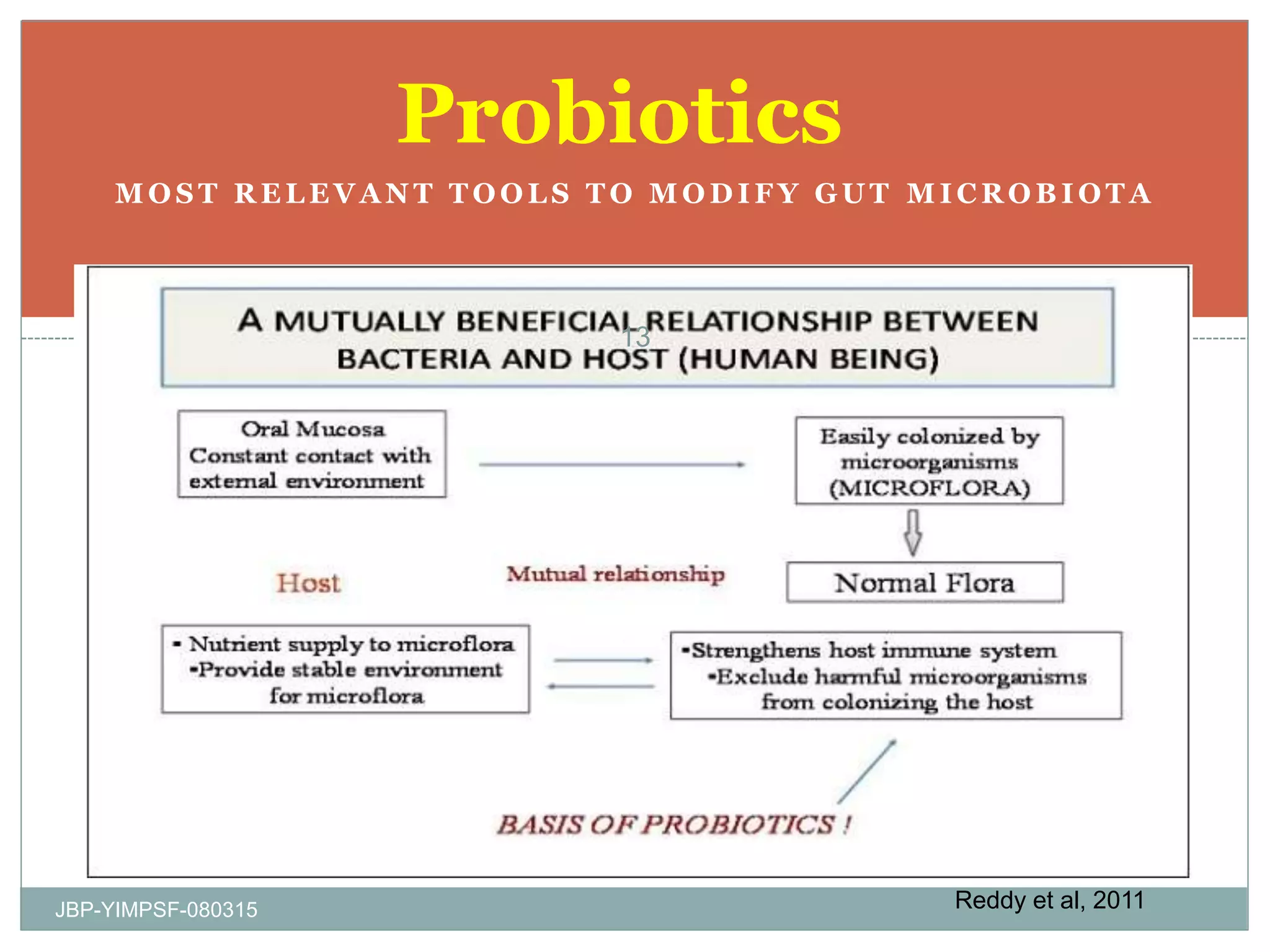
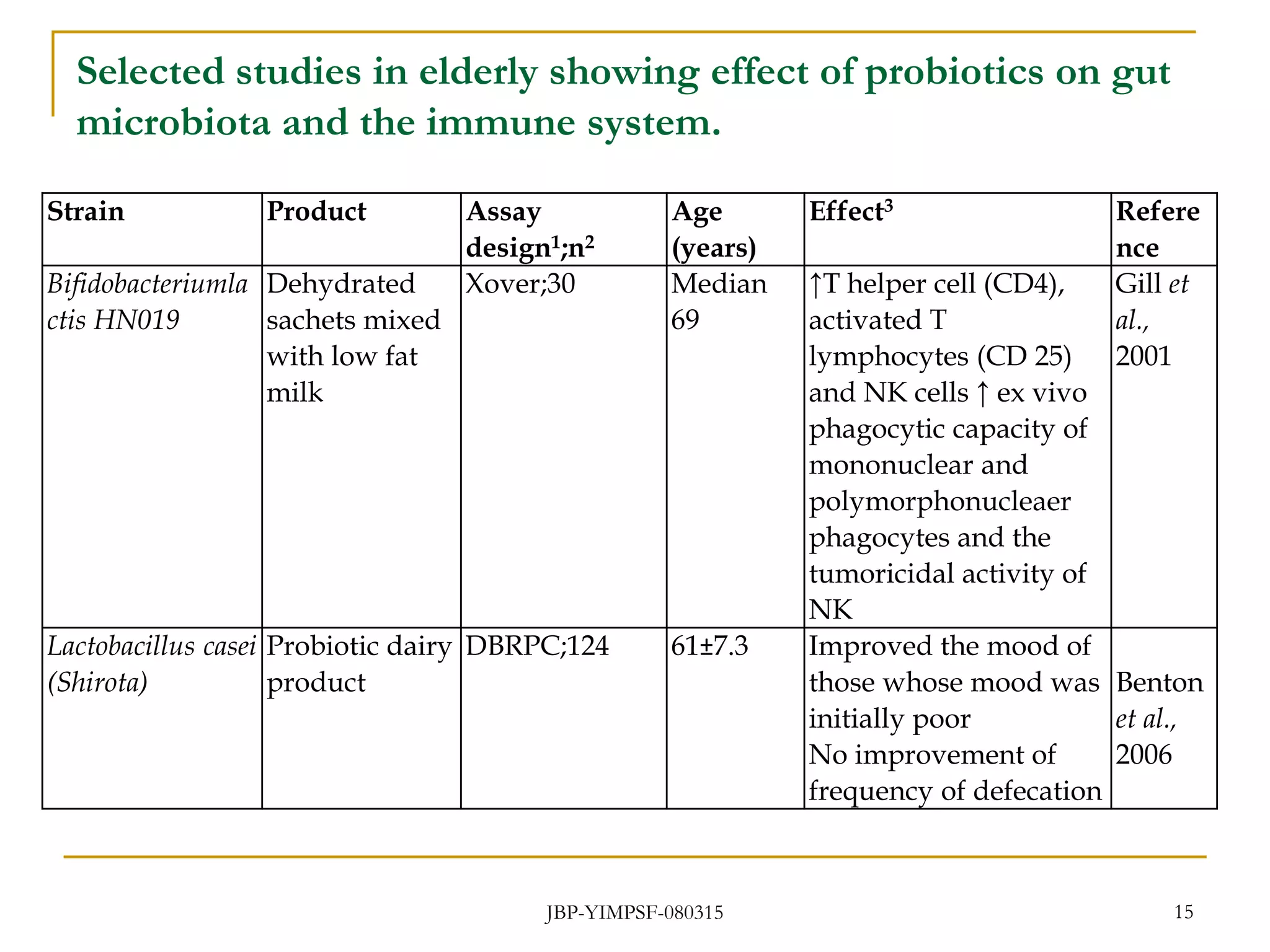
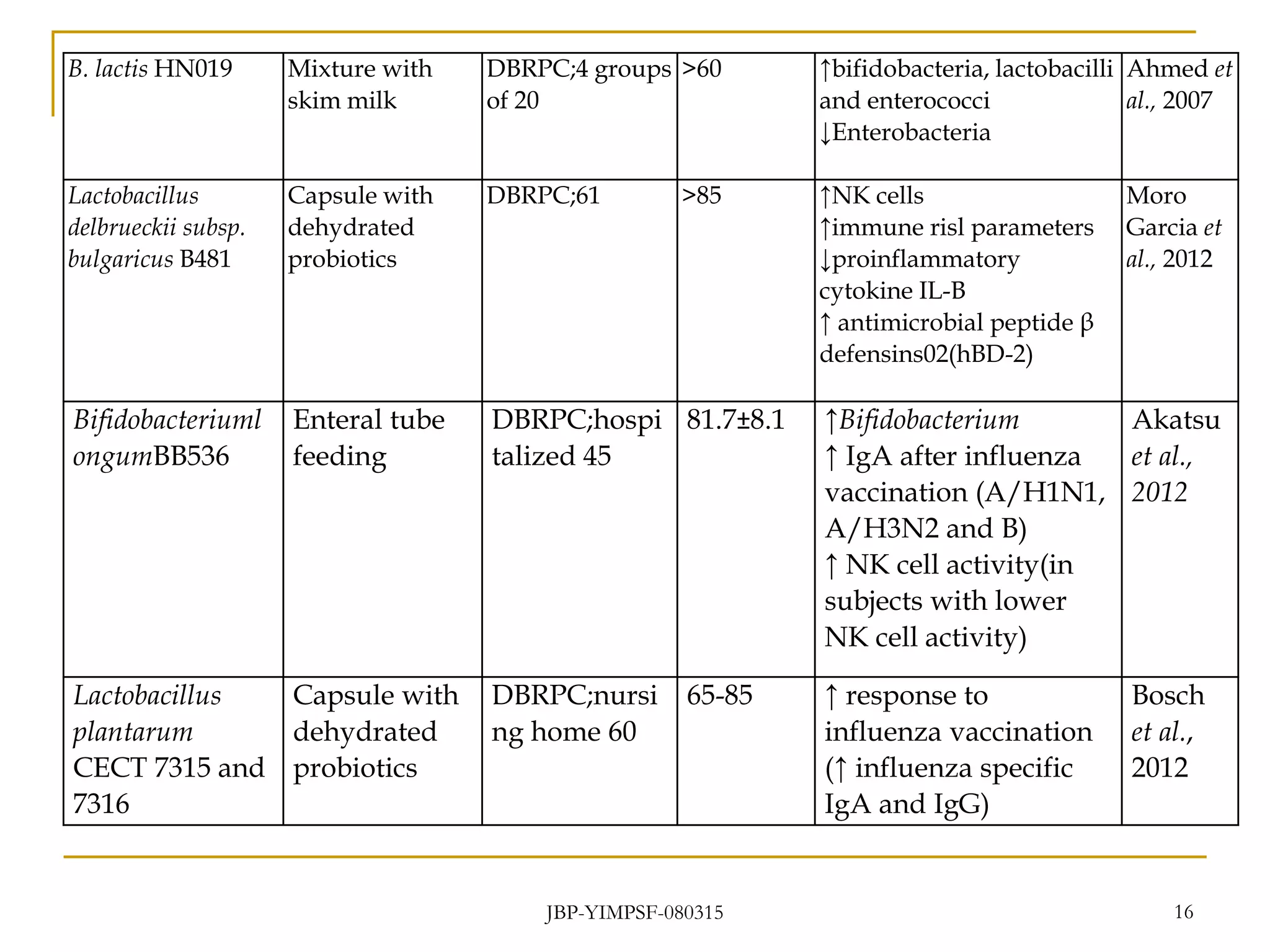
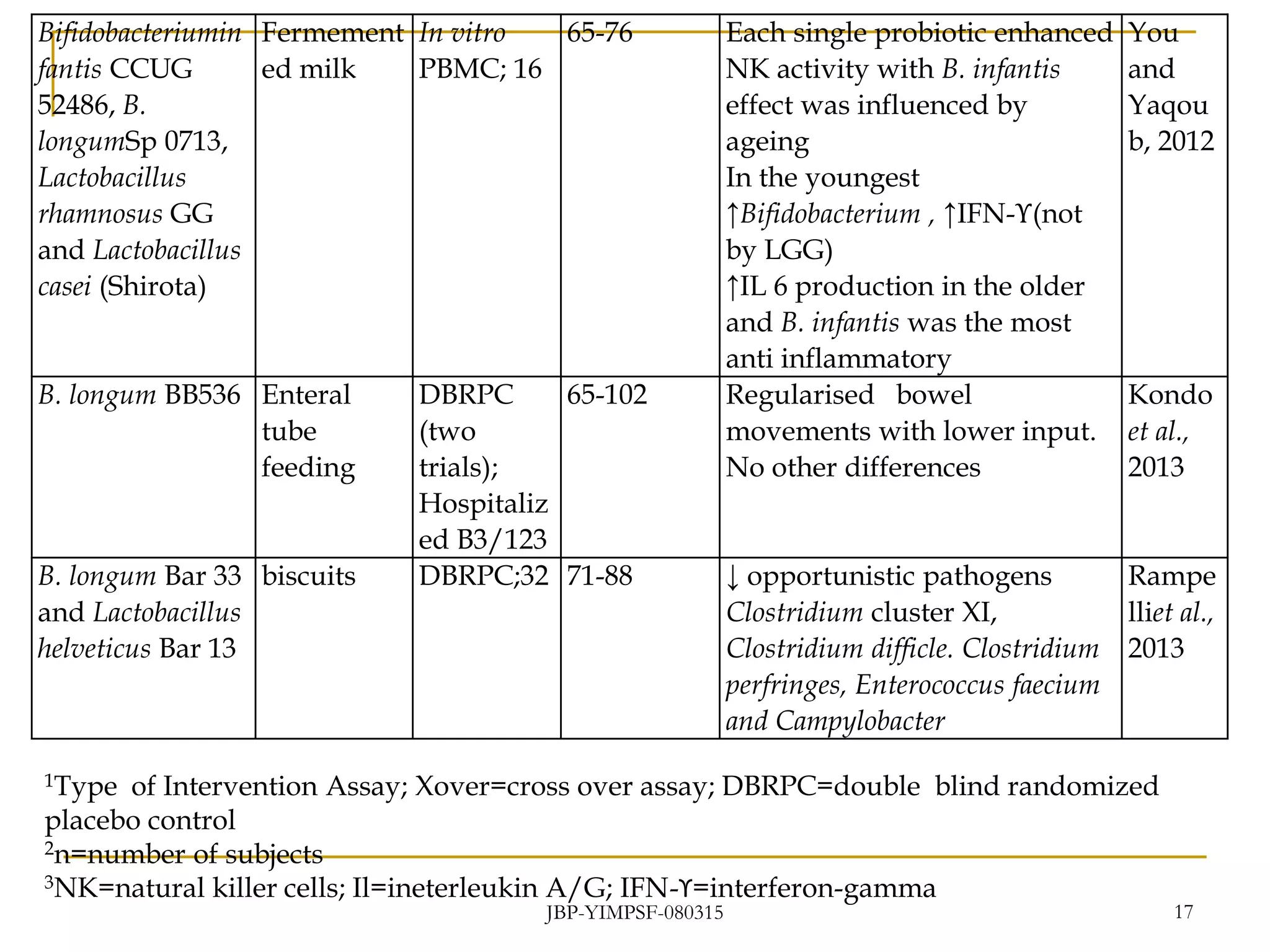
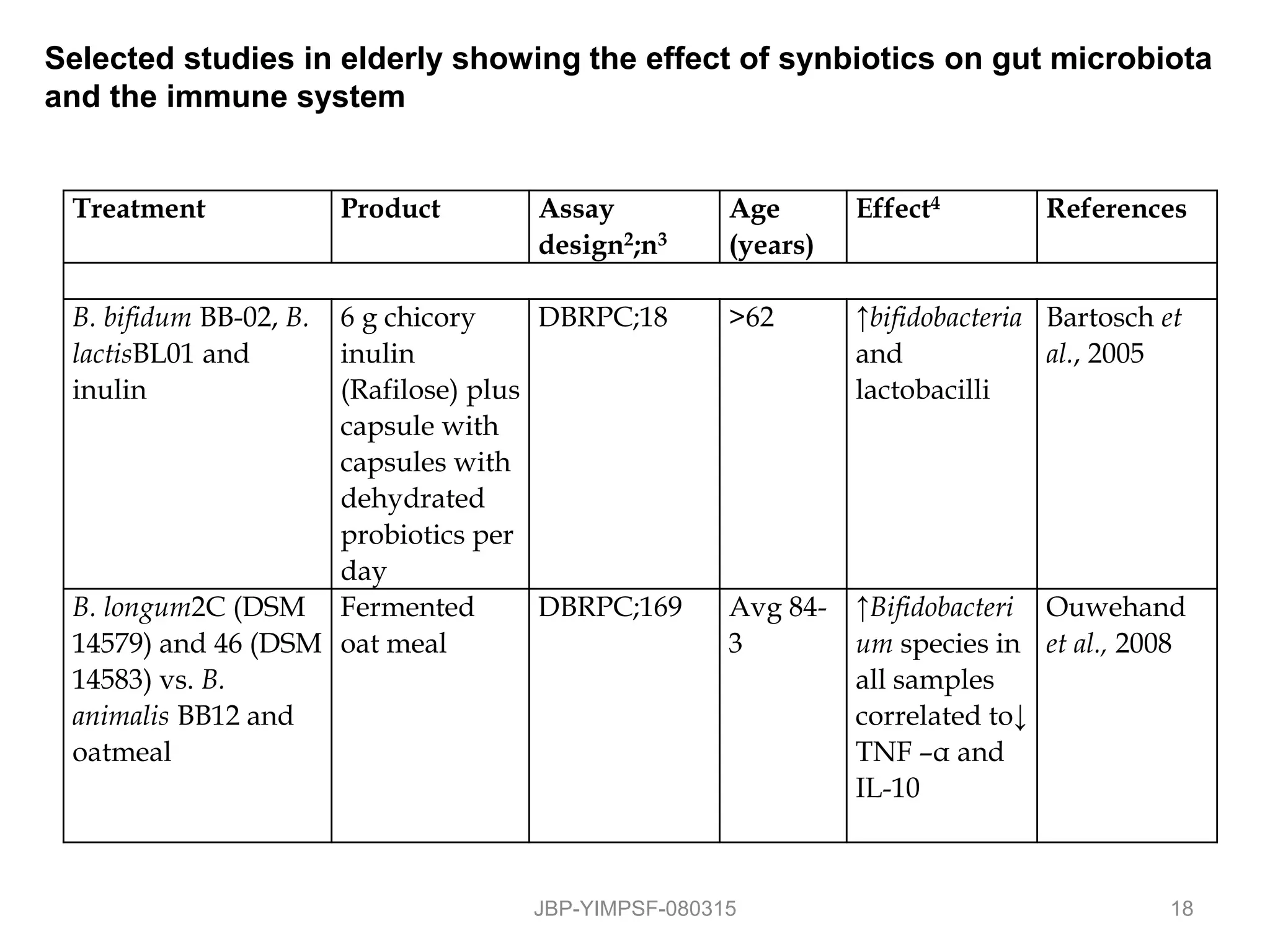
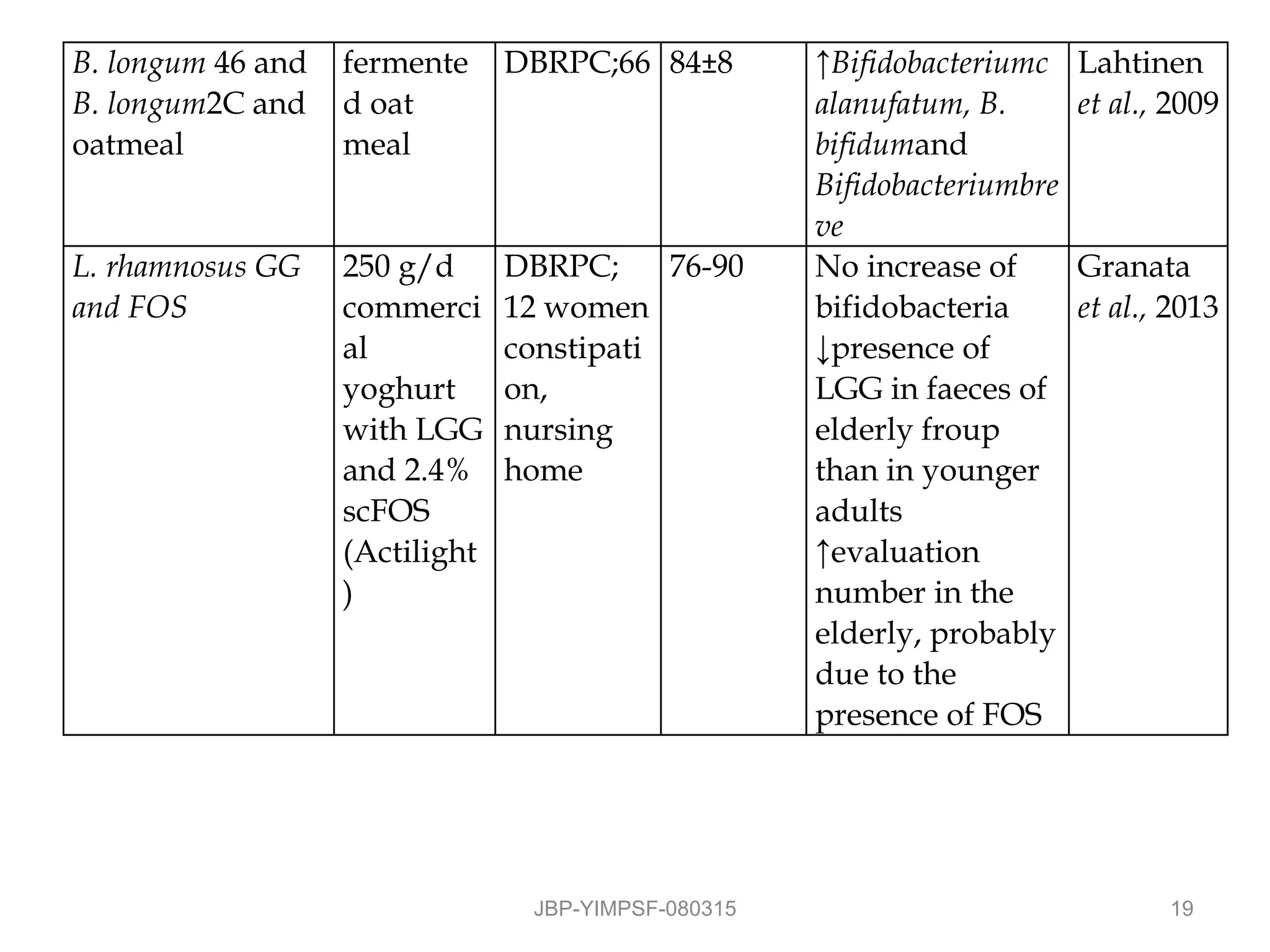
1) The document discusses the potential benefits of probiotics for the elderly population and summarizes several studies that have investigated the effects of various probiotic strains on the gut microbiota and immune system of elderly subjects.
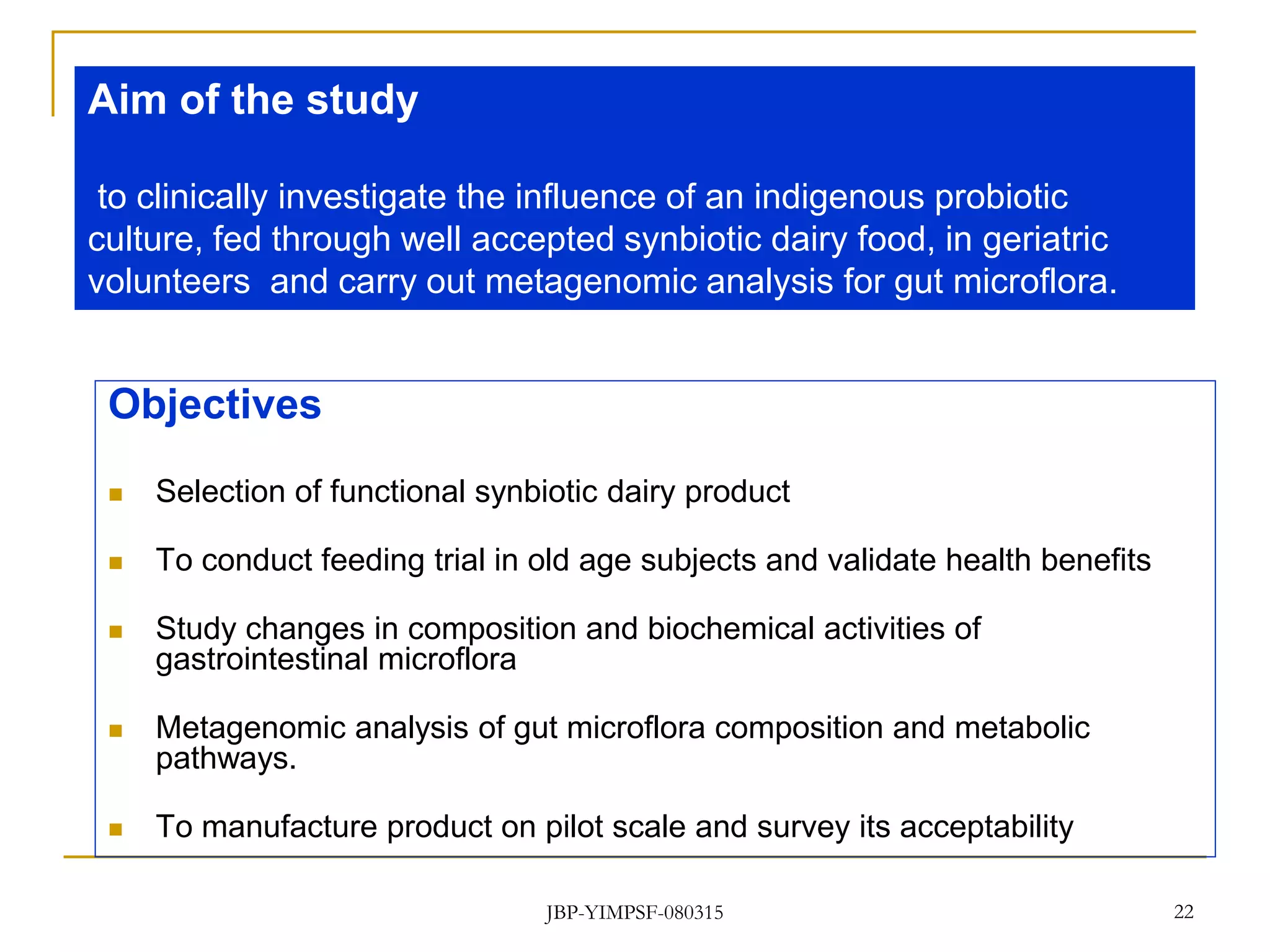
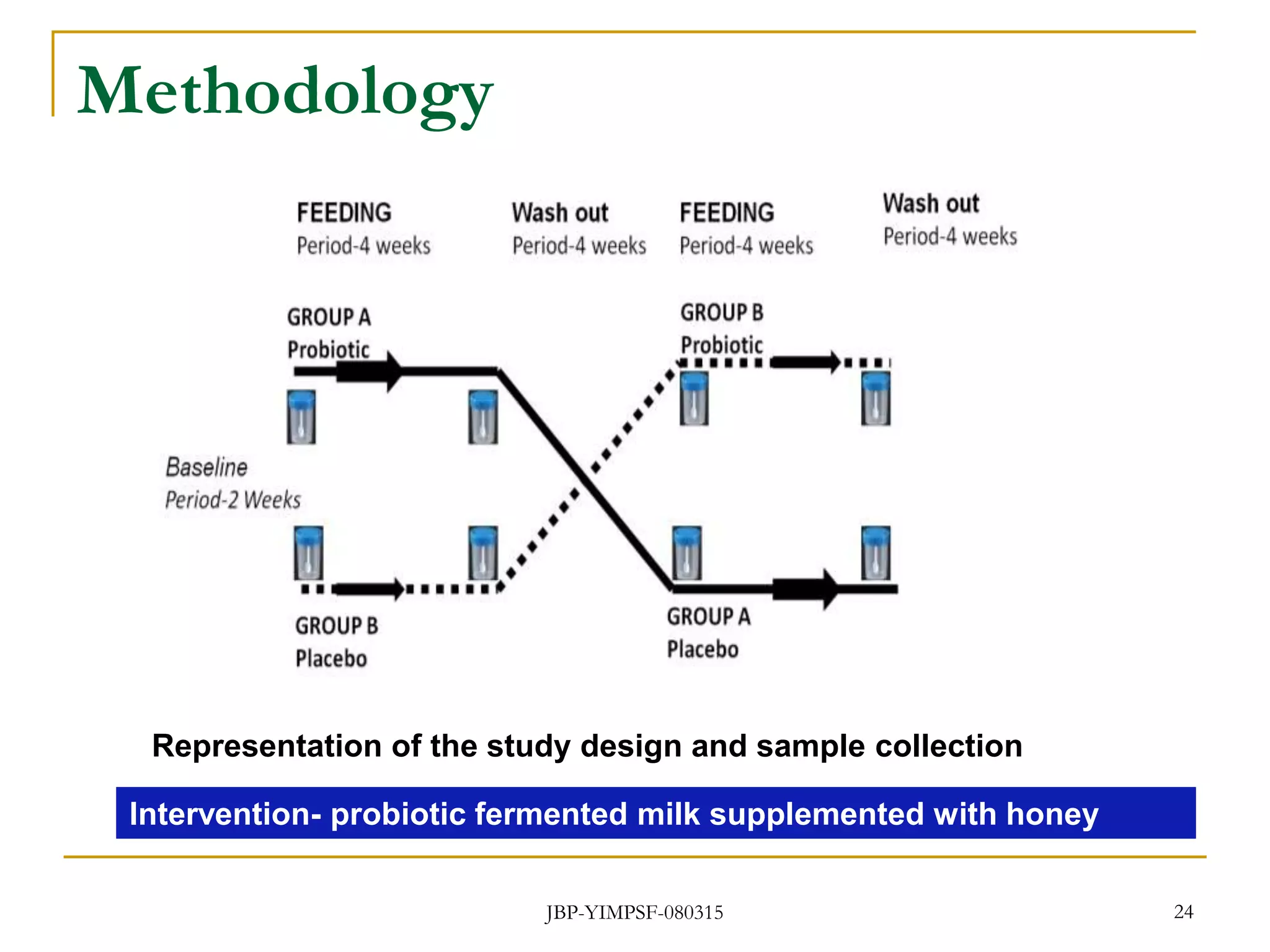
2) It then outlines objectives and methodology for a proposed clinical study to investigate the influence of the probiotic Lactobacillus helveticus MTCC 5463, delivered through a synbiotic dairy product, on the gut microbiota and health of geriatric volunteers.
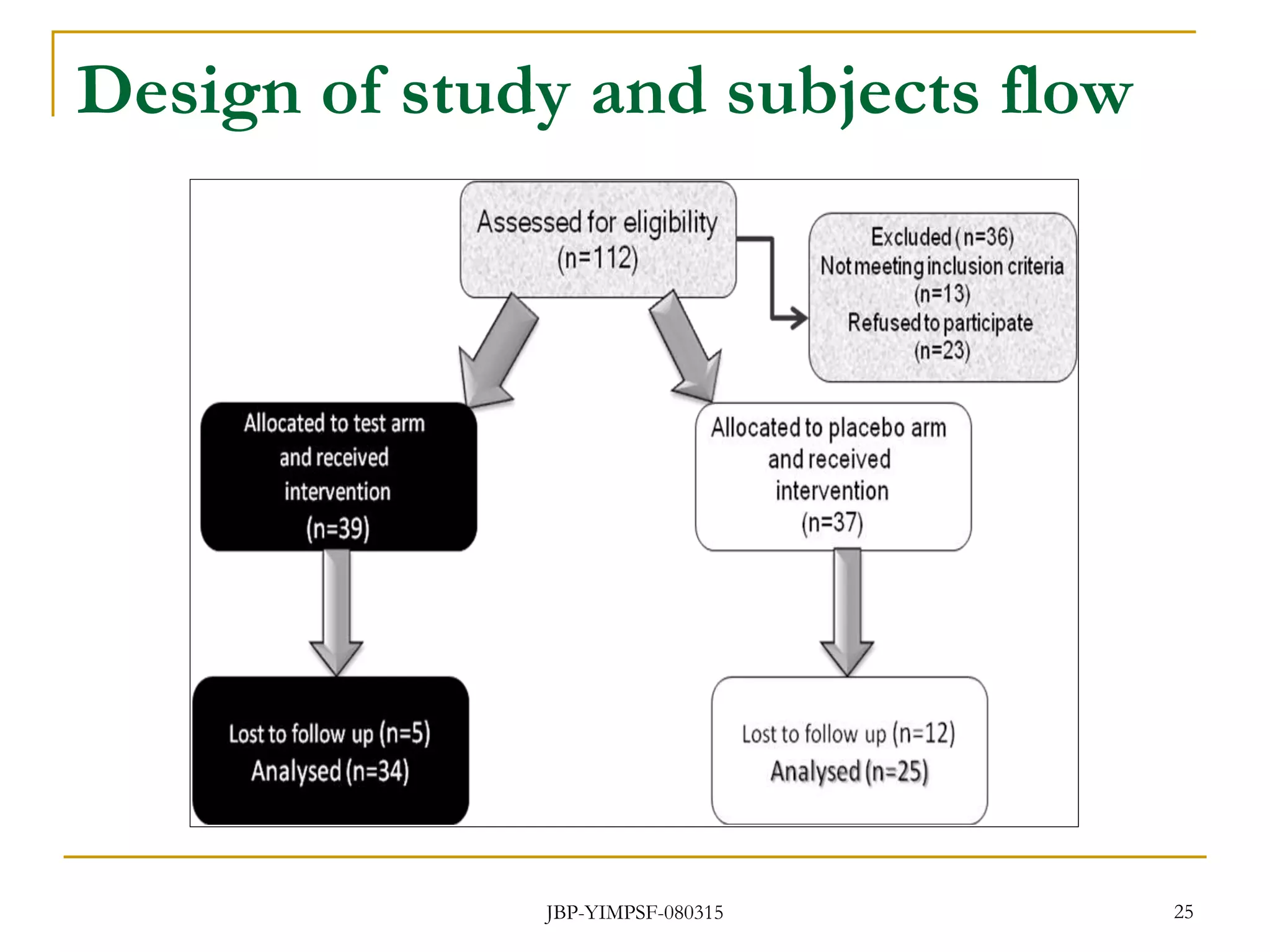
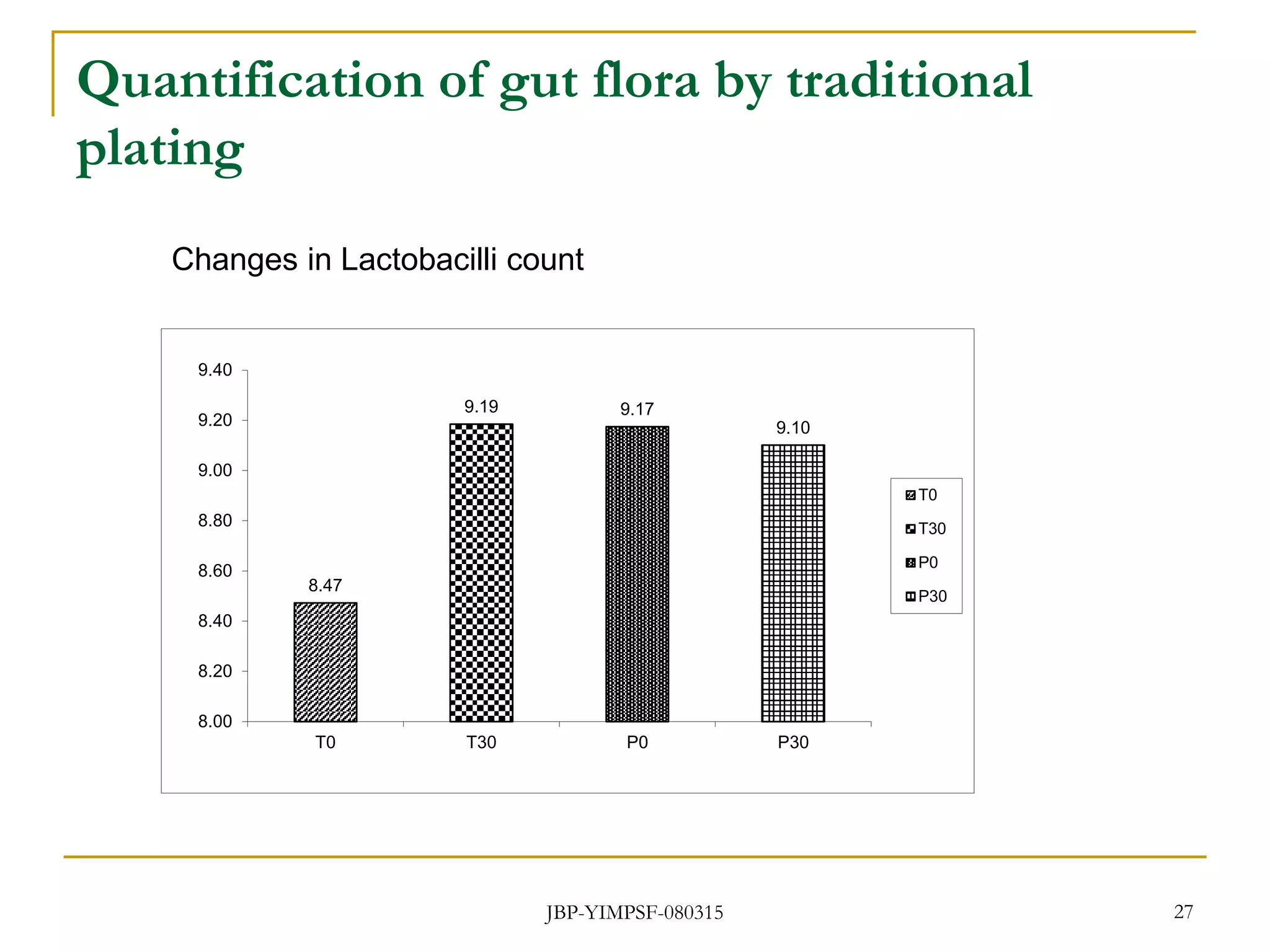
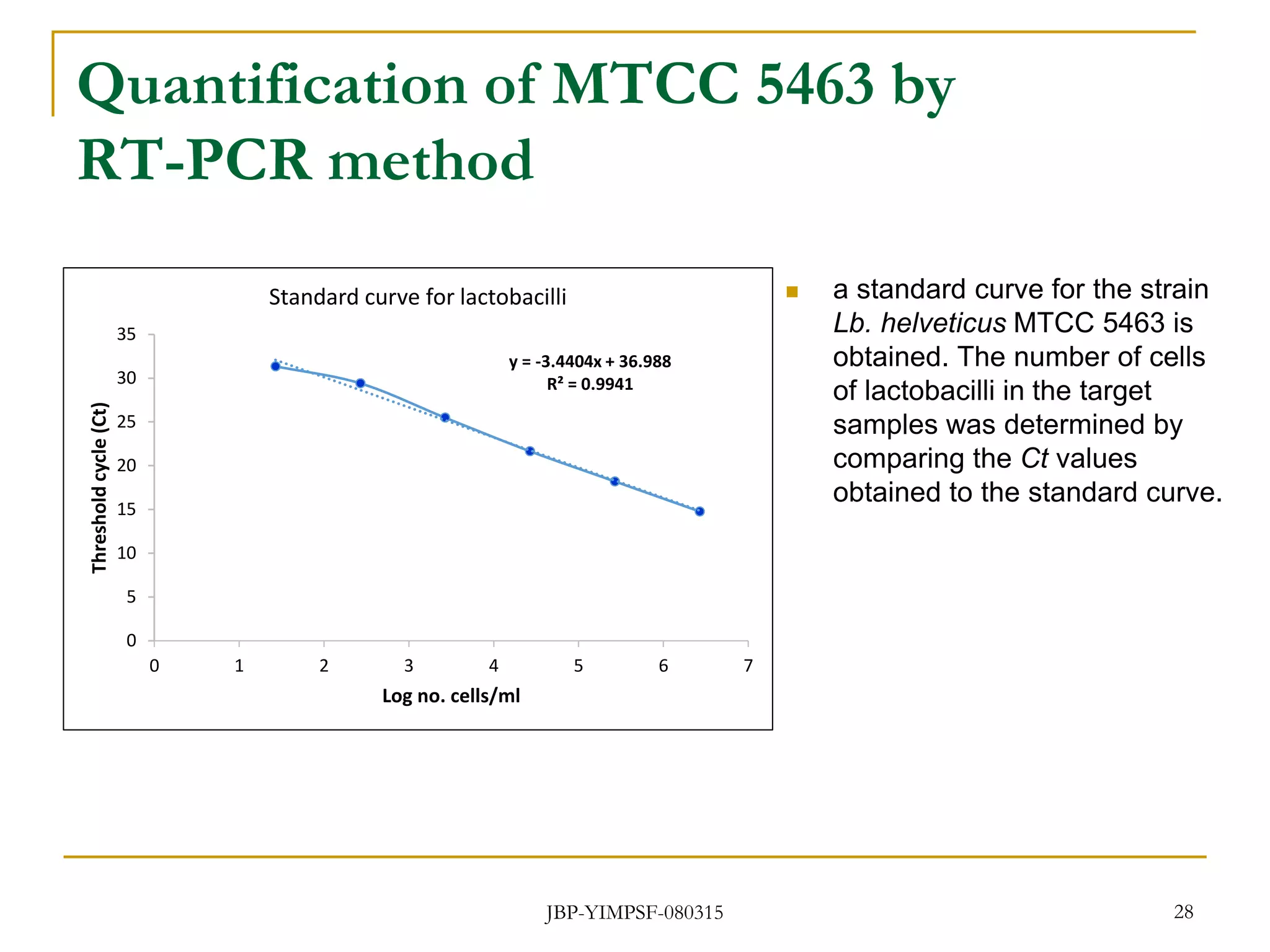
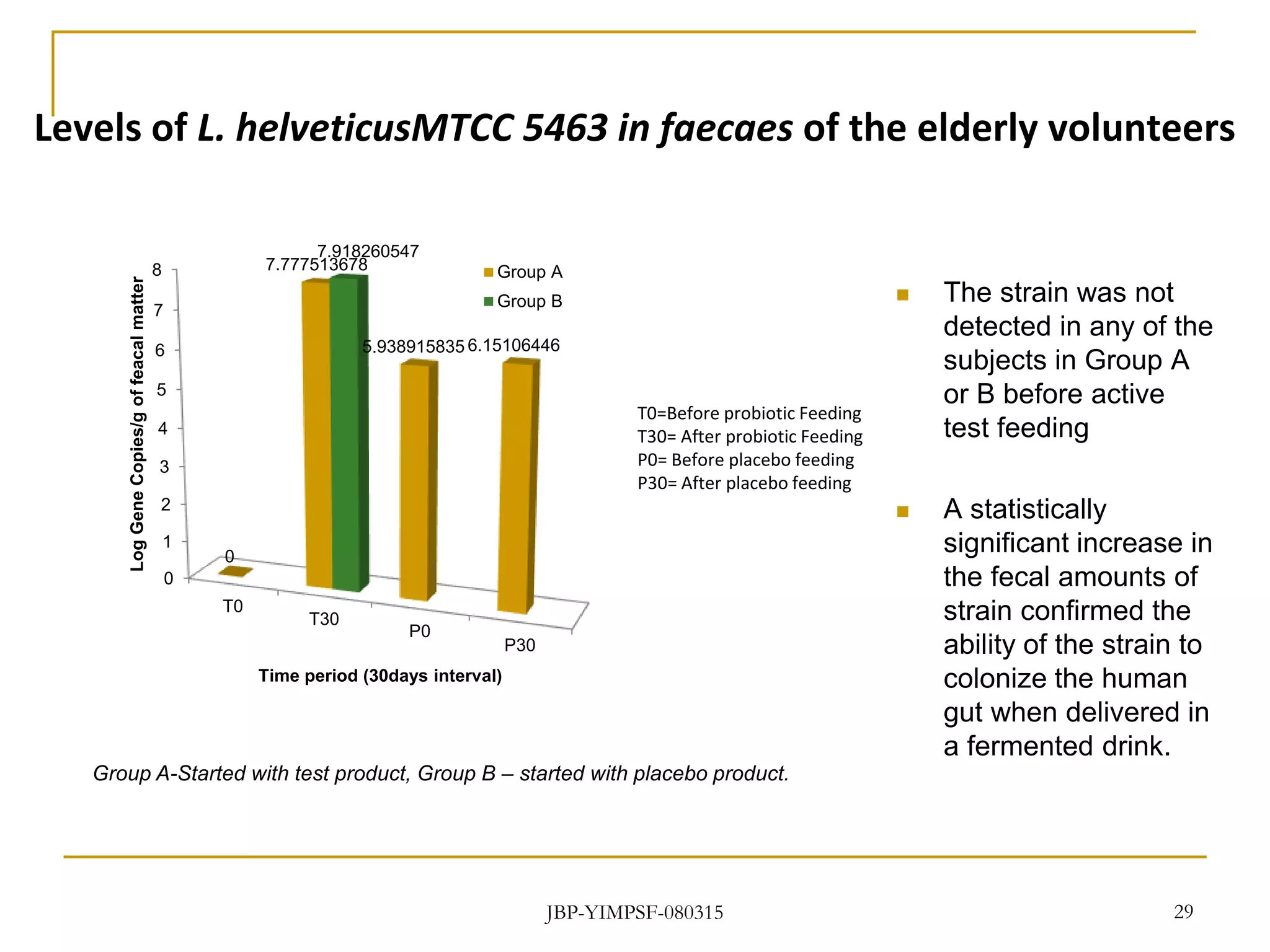
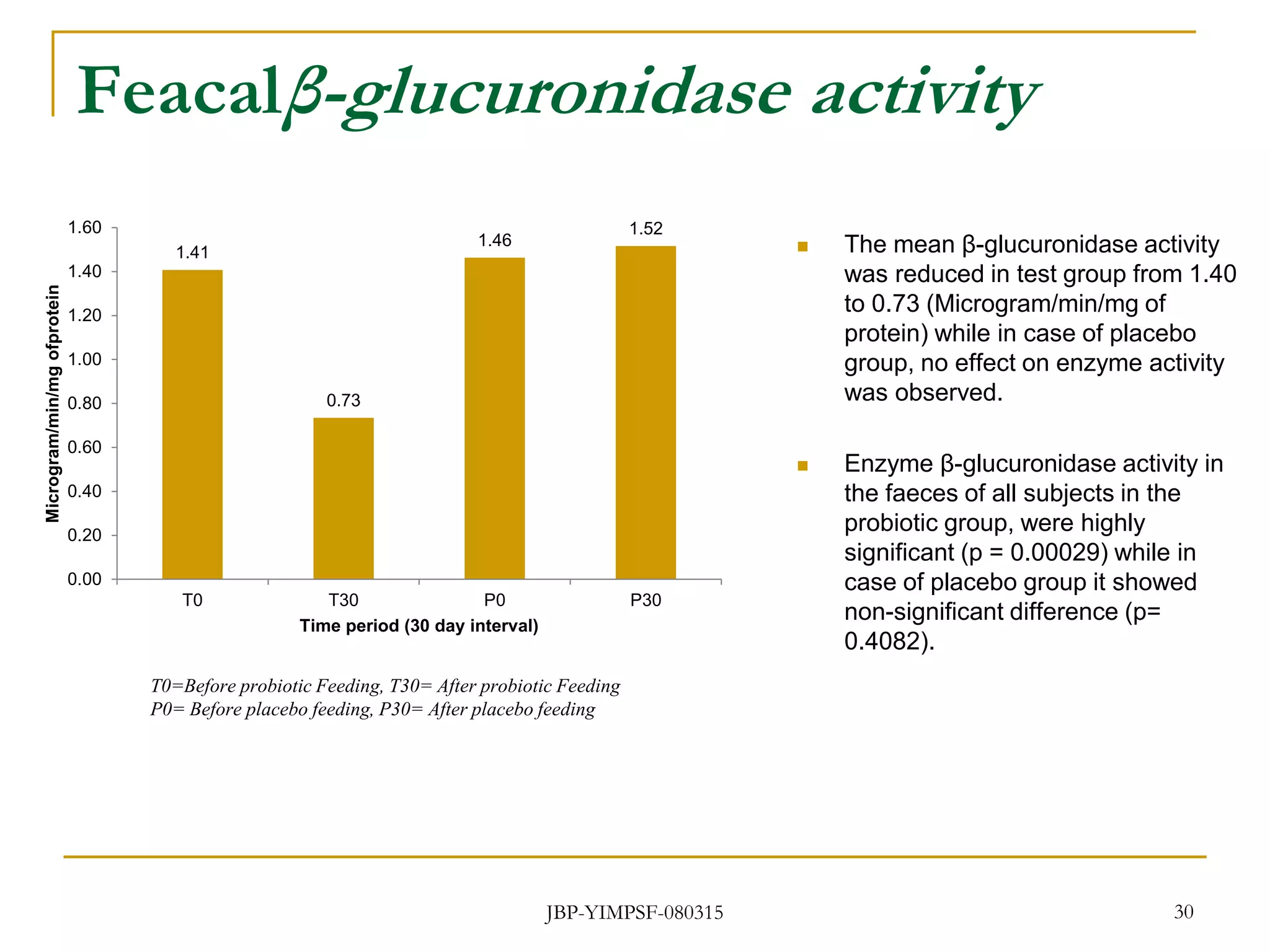
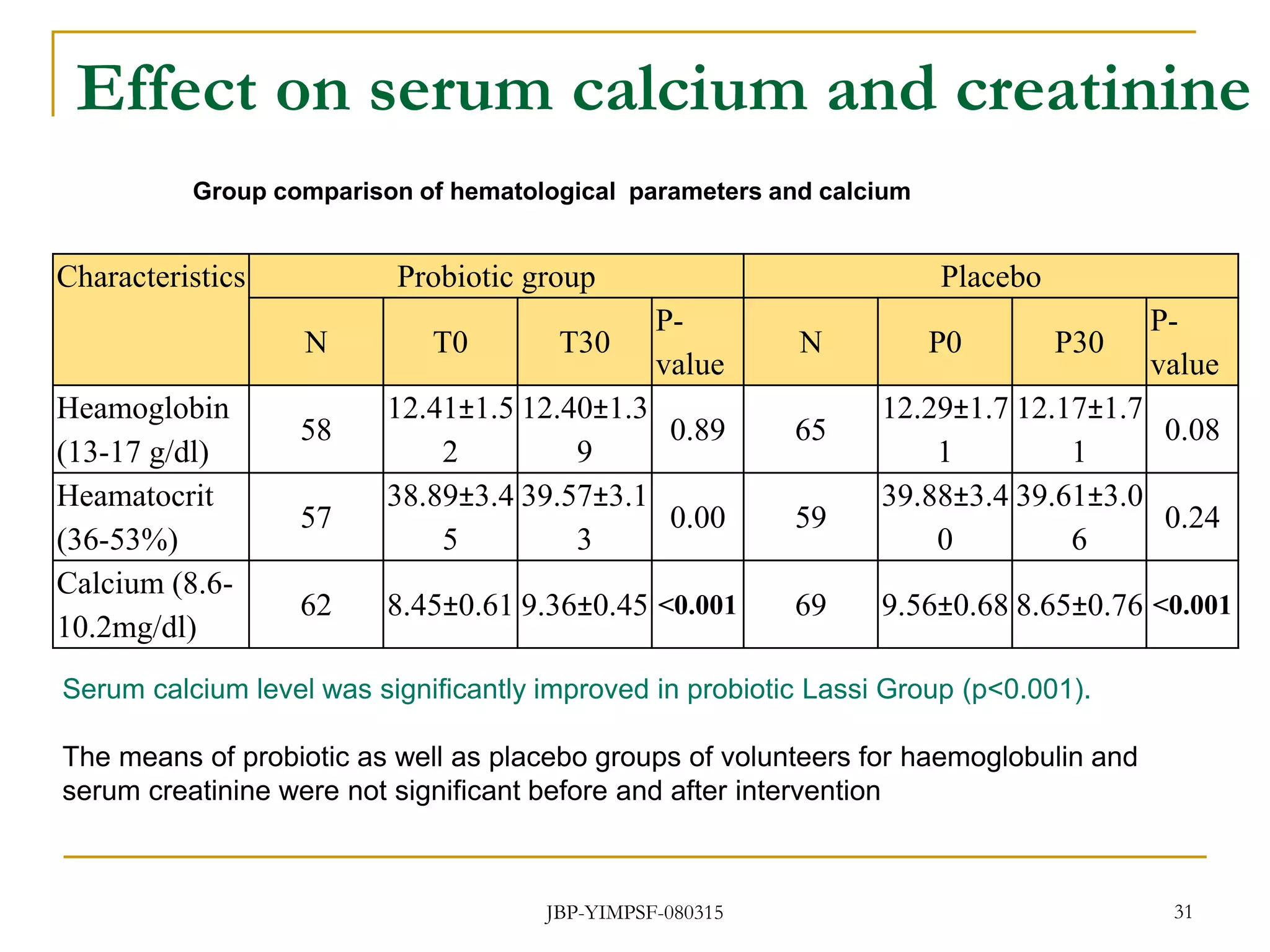
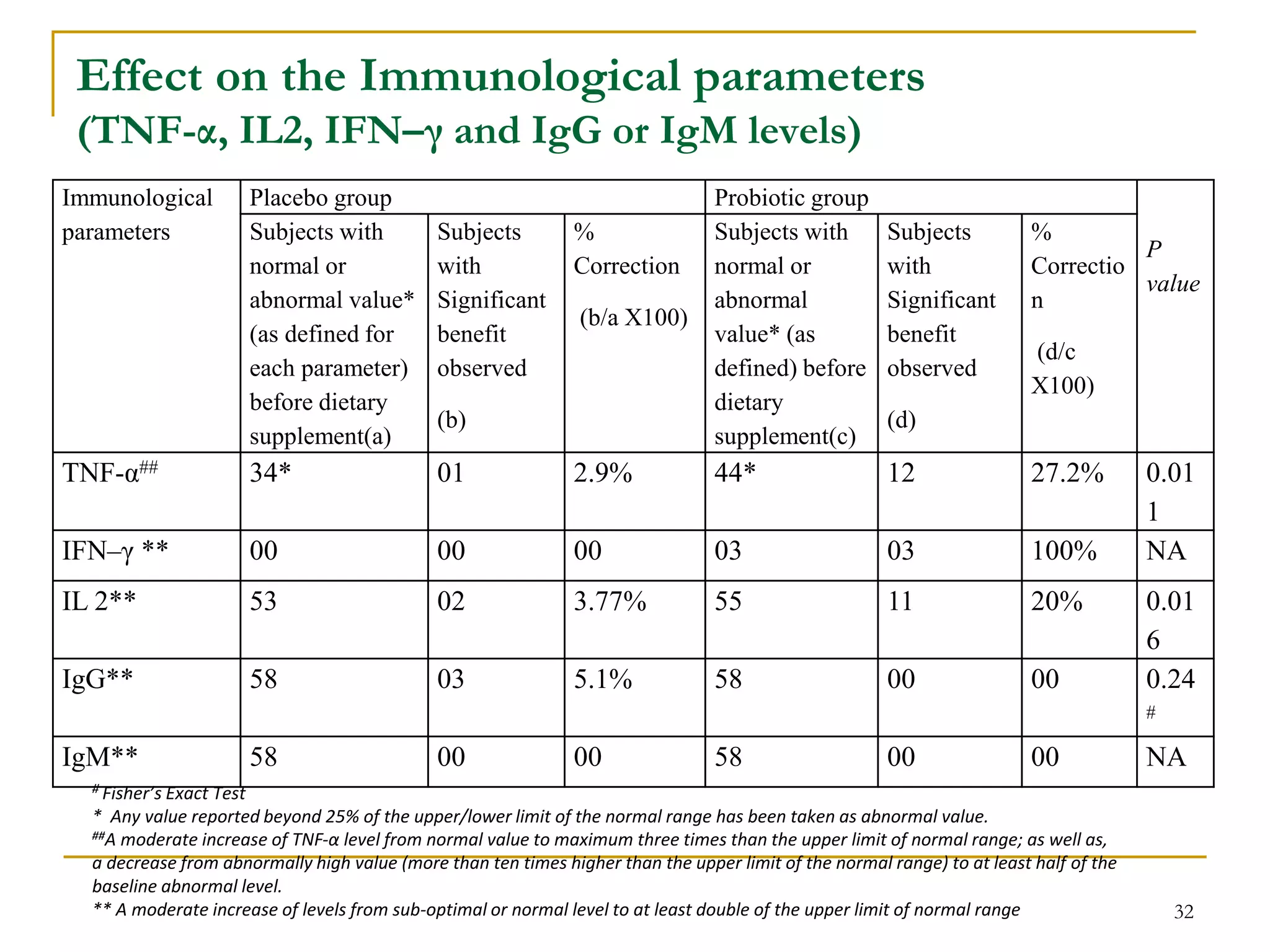
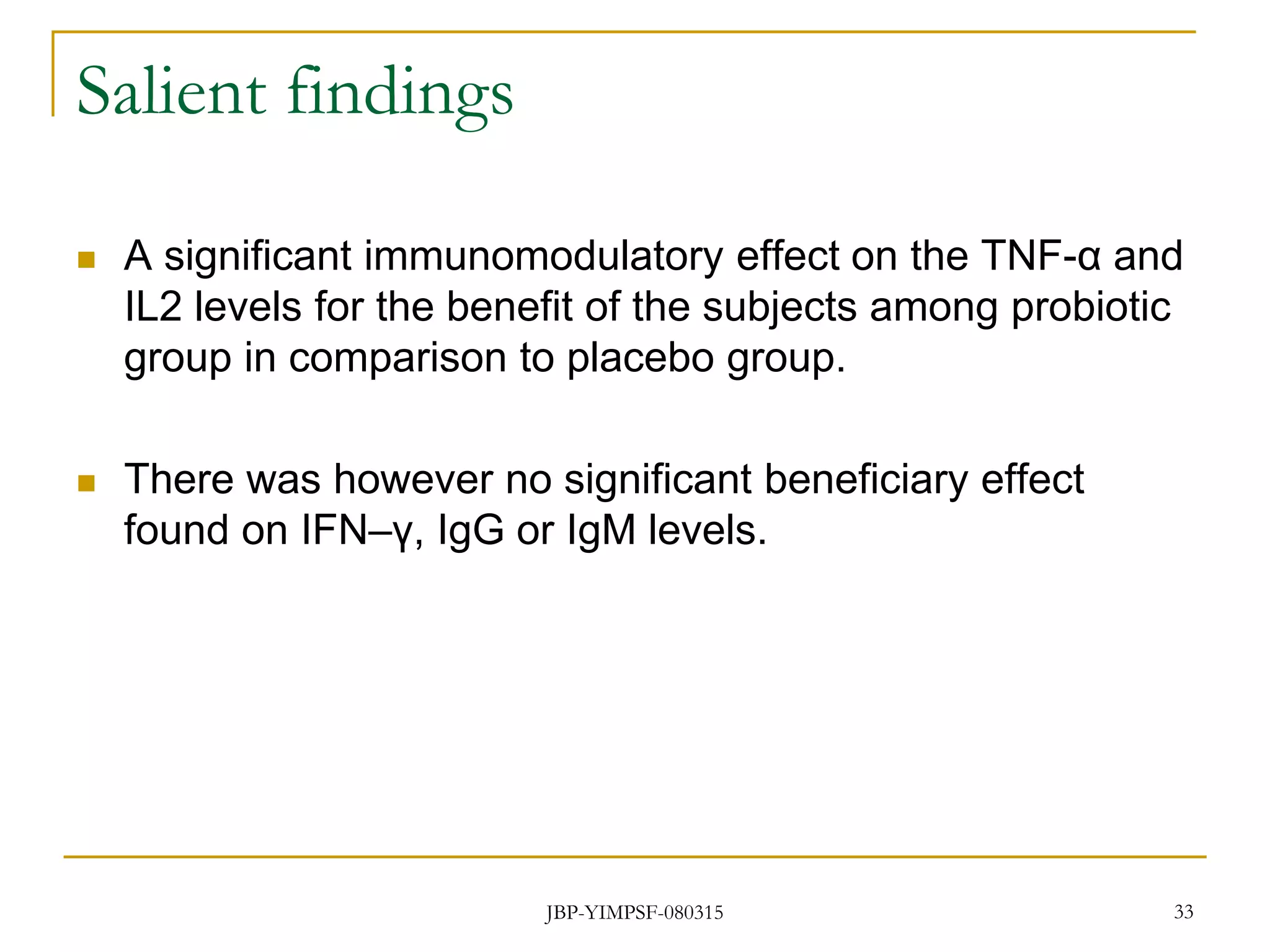
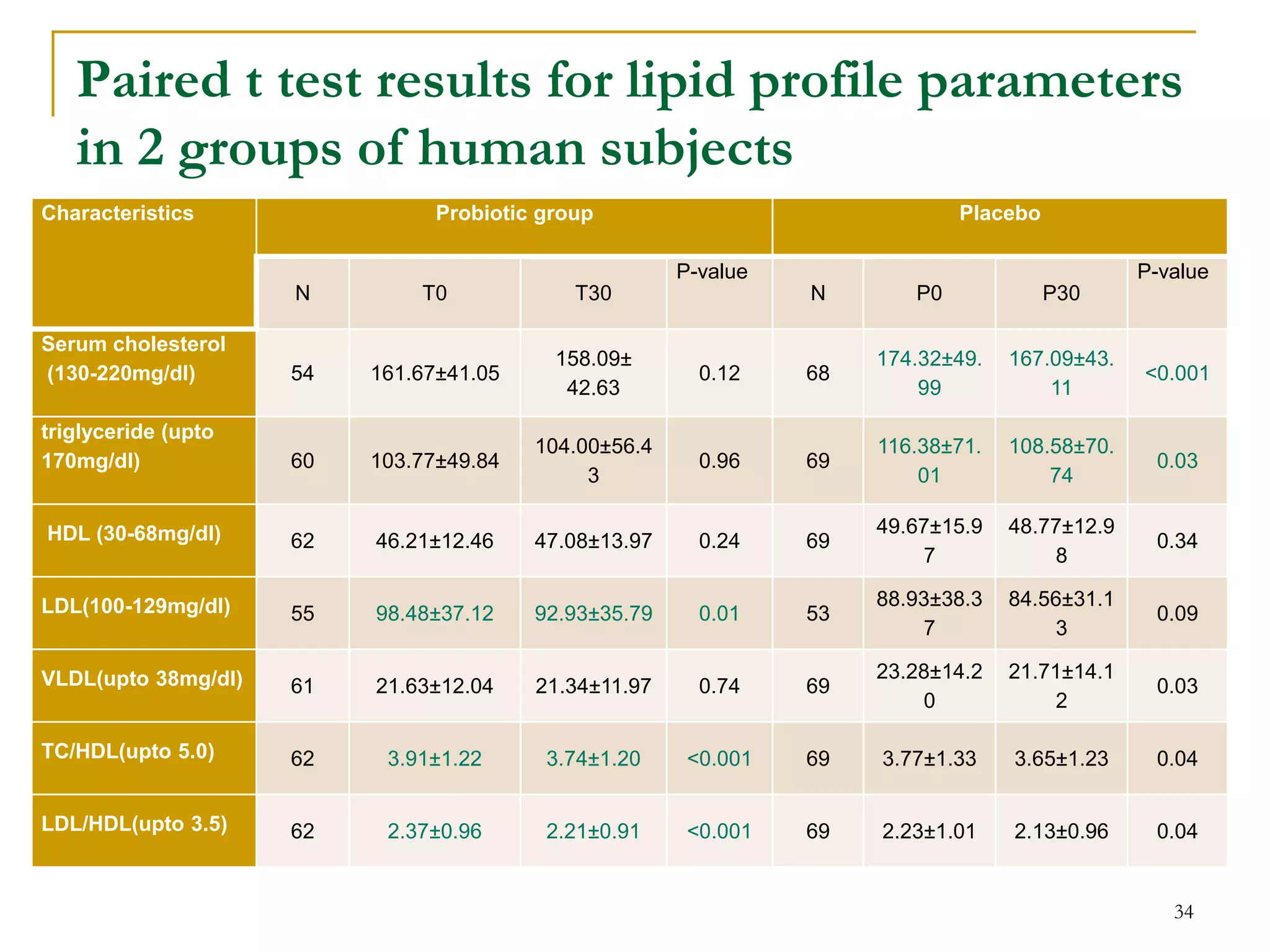
3) The study will involve collecting fecal samples and blood to analyze changes in gut microflora composition, biochemical activities, and hematological and immunological parameters before and after consumption of the synbiotic product.