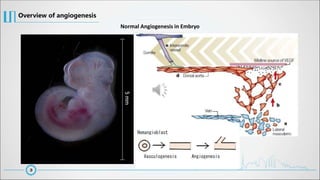
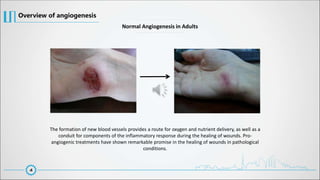
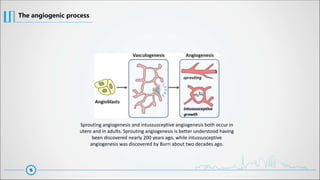
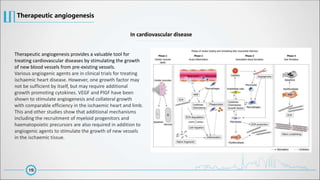
The document provides a comprehensive overview of angiogenesis, detailing its physiological role in blood vessel formation, regulation through various activators and inhibitors, and therapeutic applications in health and disease. It explains two primary processes—sprouting and intussusceptive angiogenesis—and discusses the significance of the vascular endothelial growth factor (VEGF) in mediating these processes. Additionally, it touches on anti-angiogenic therapies targeting conditions like cancer, hemangiomas, and ocular neovascularization, highlighting ongoing clinical developments.