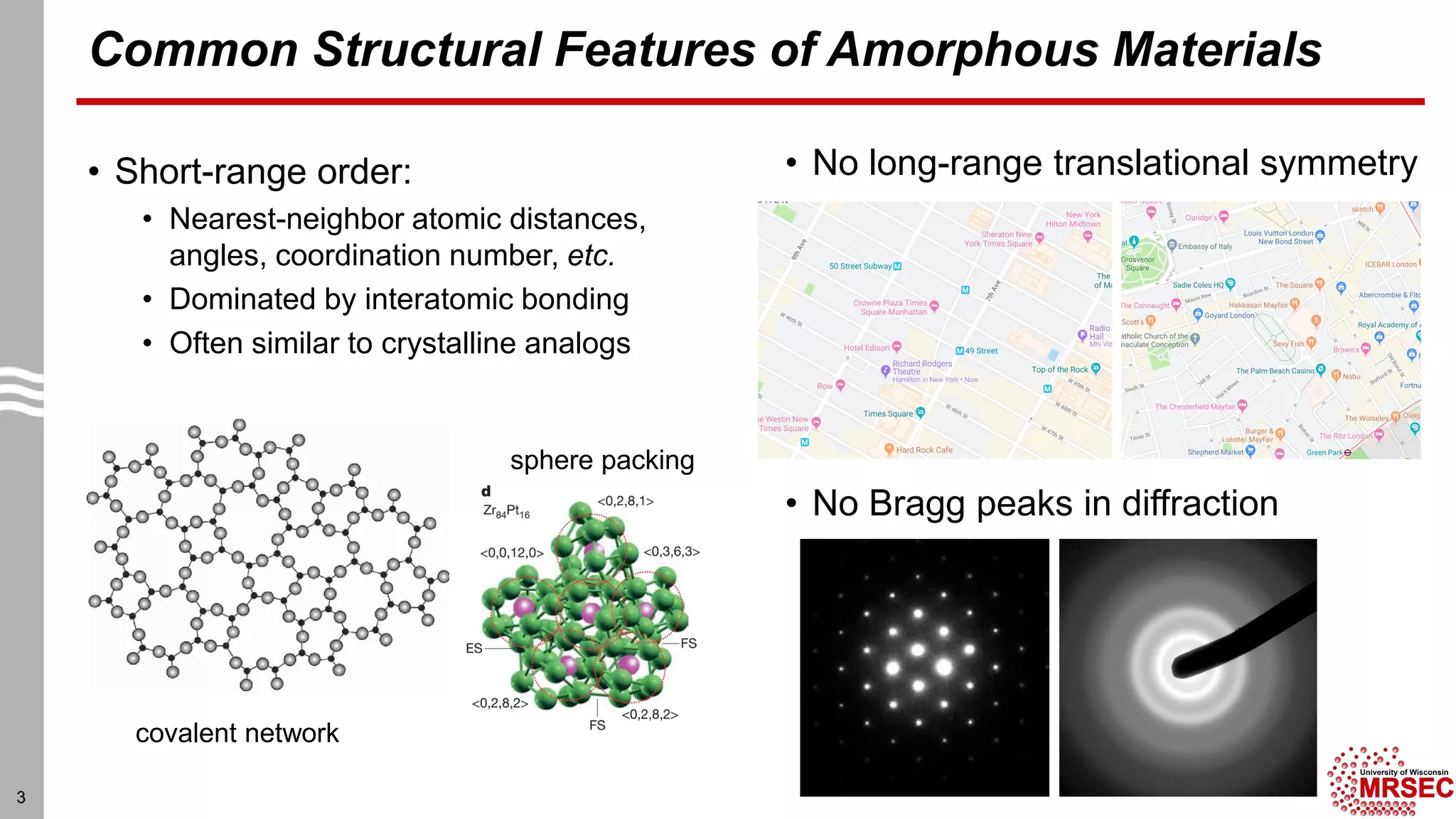
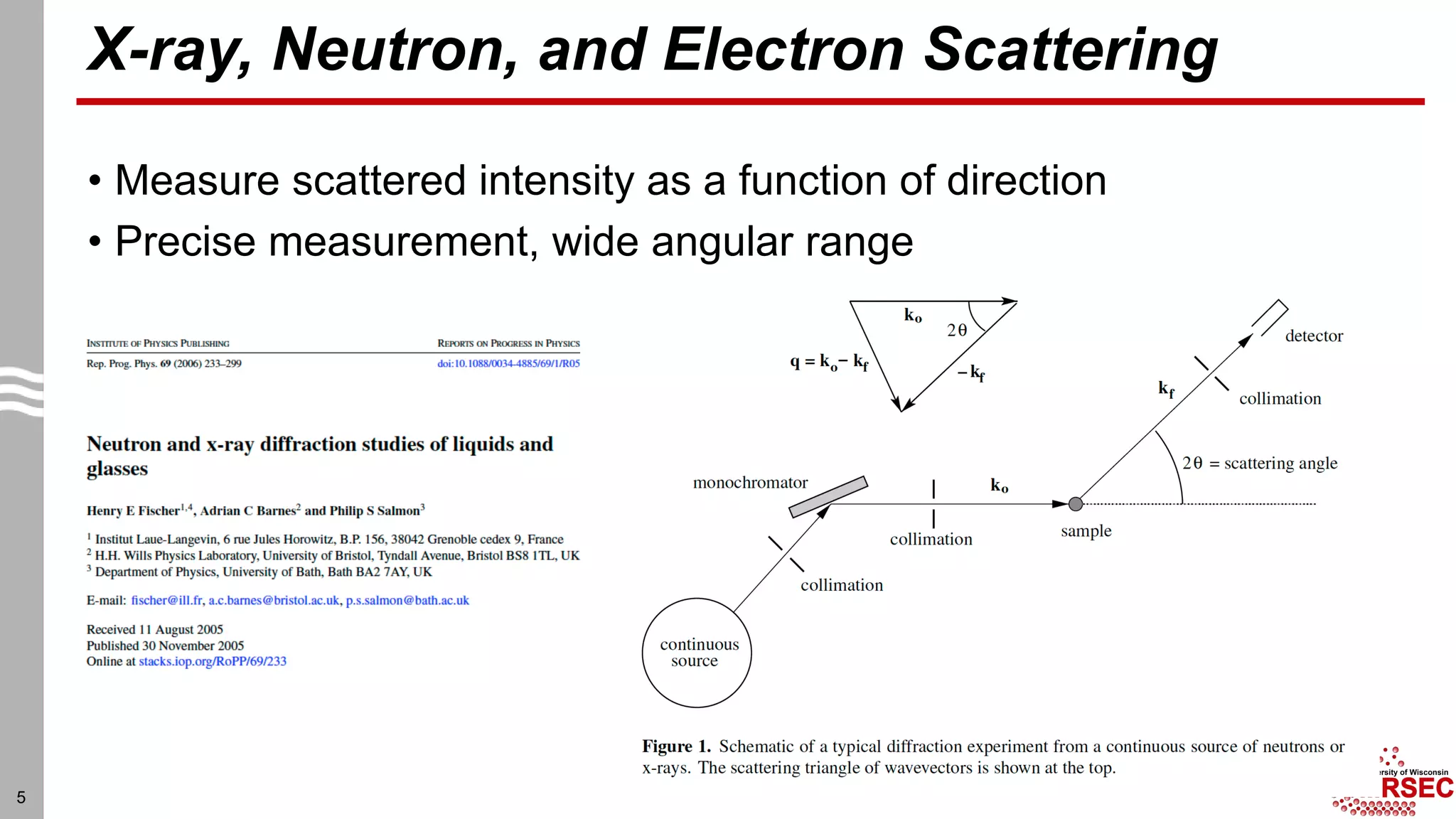
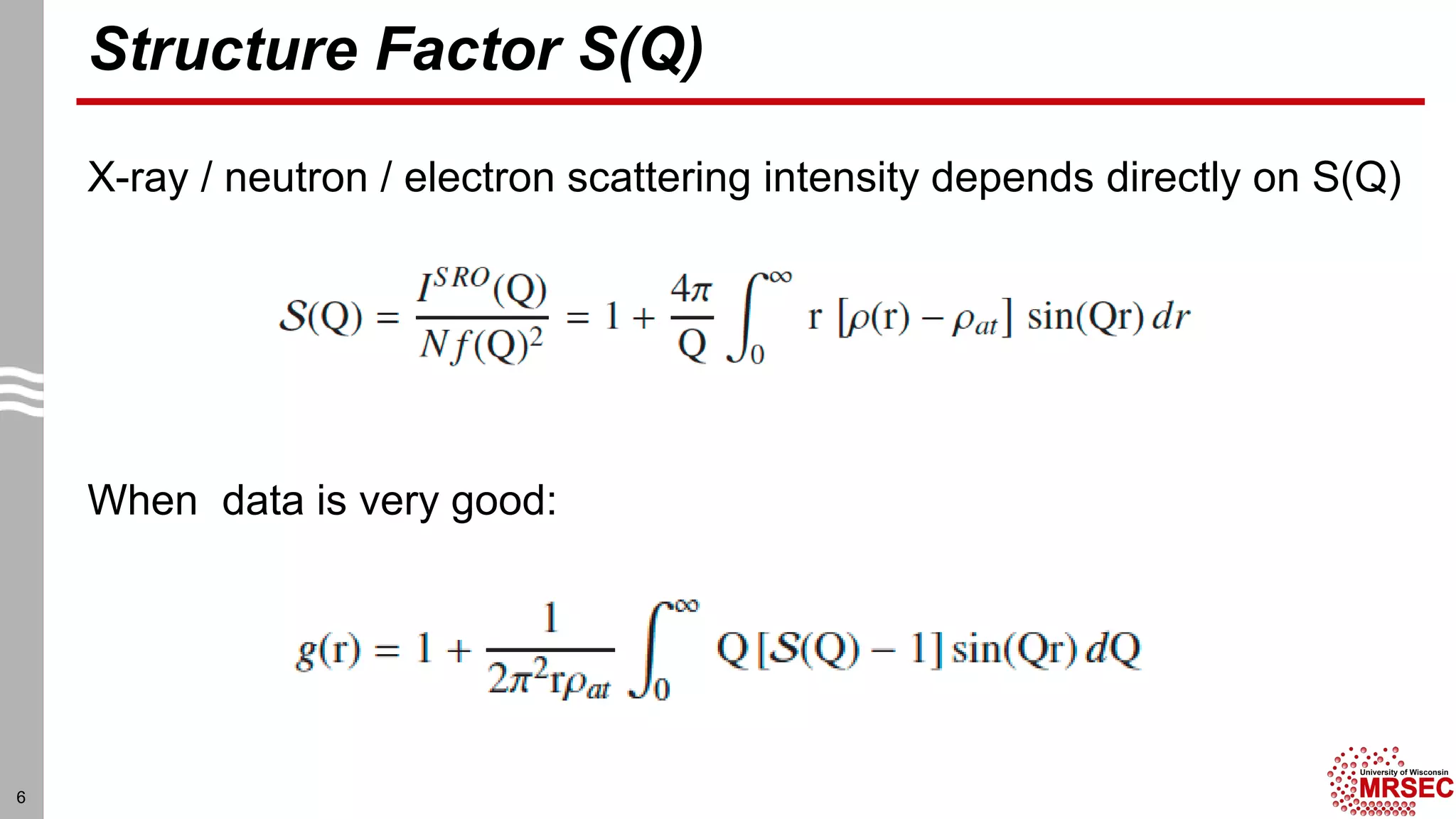
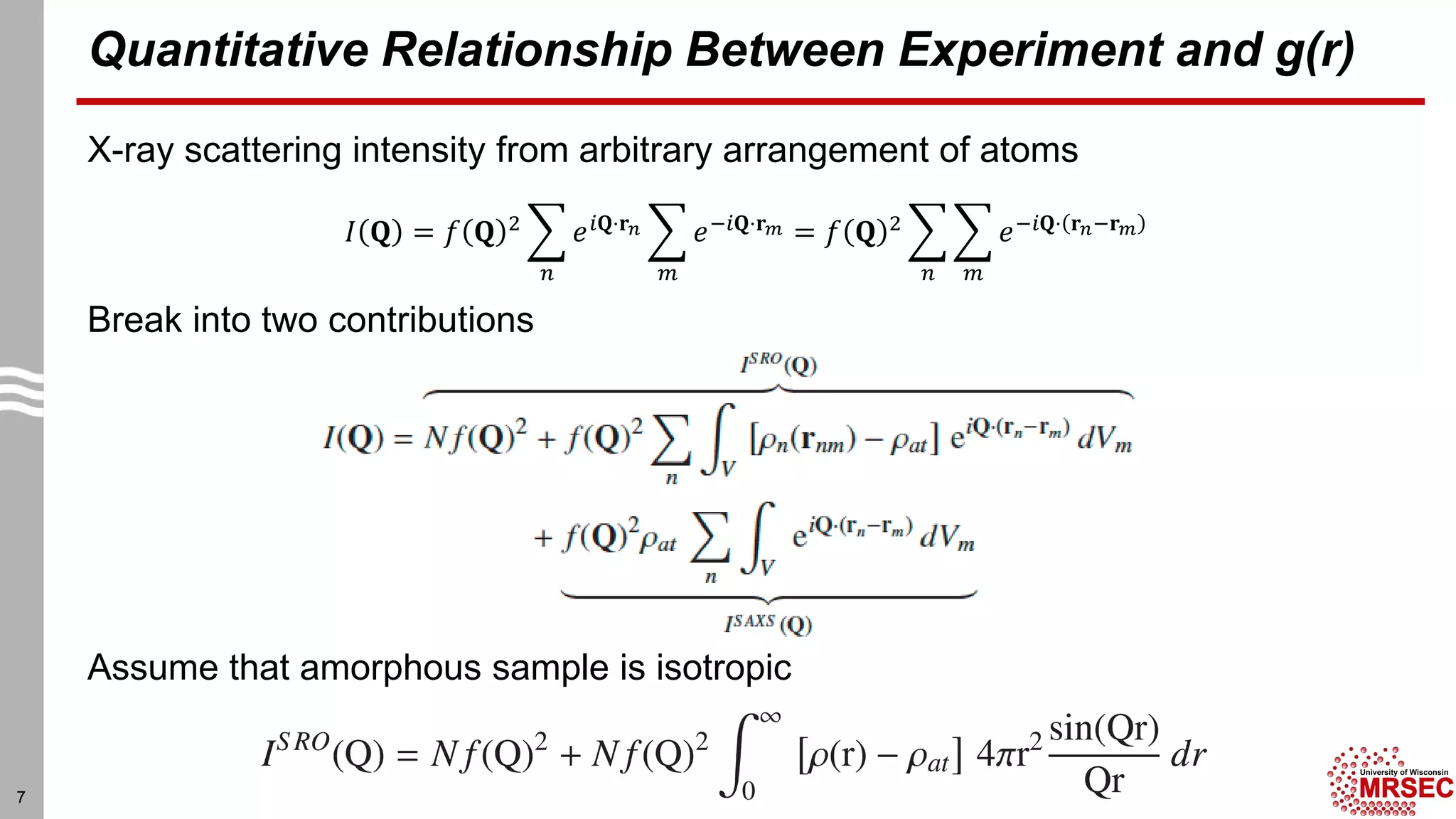
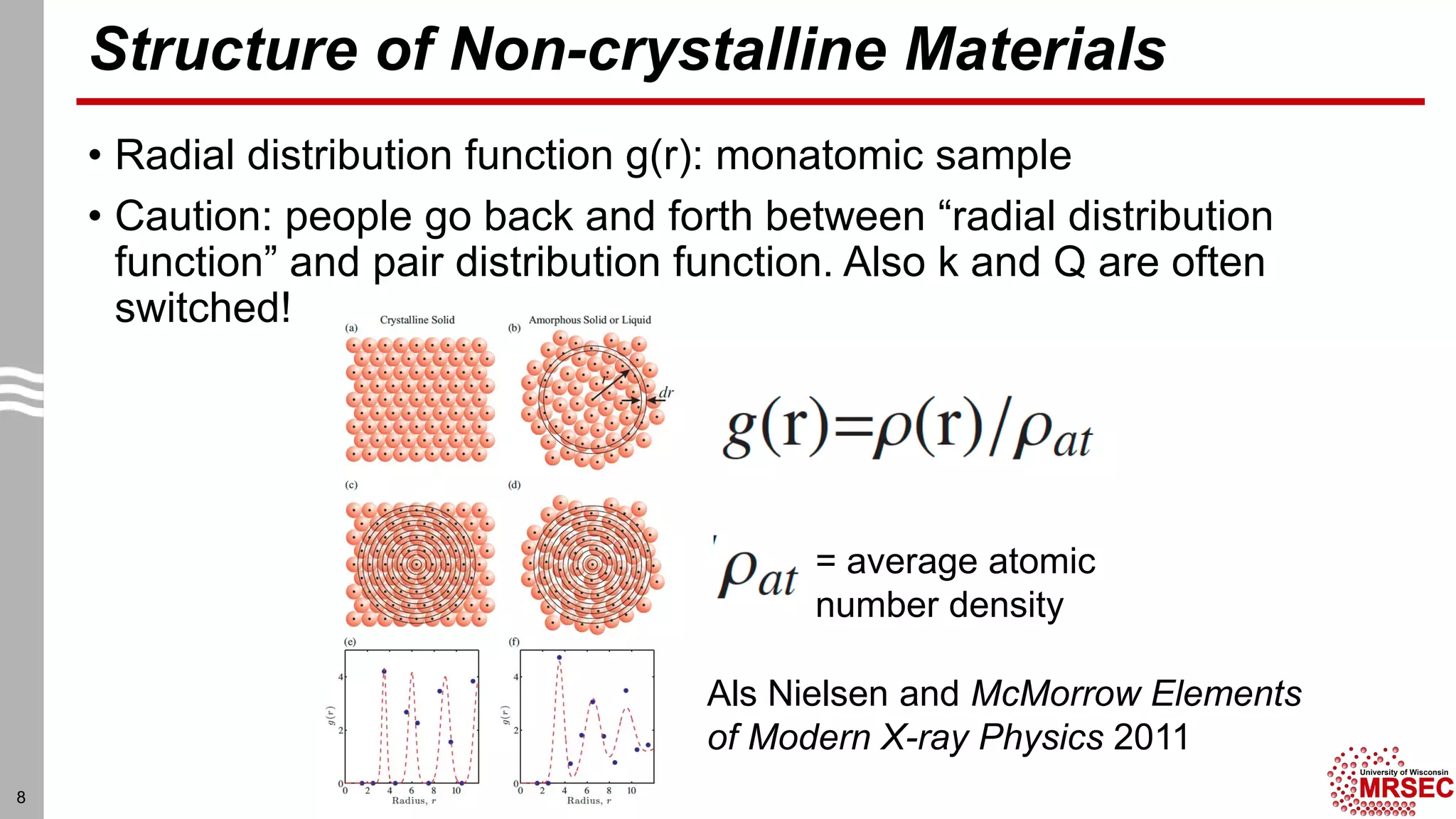
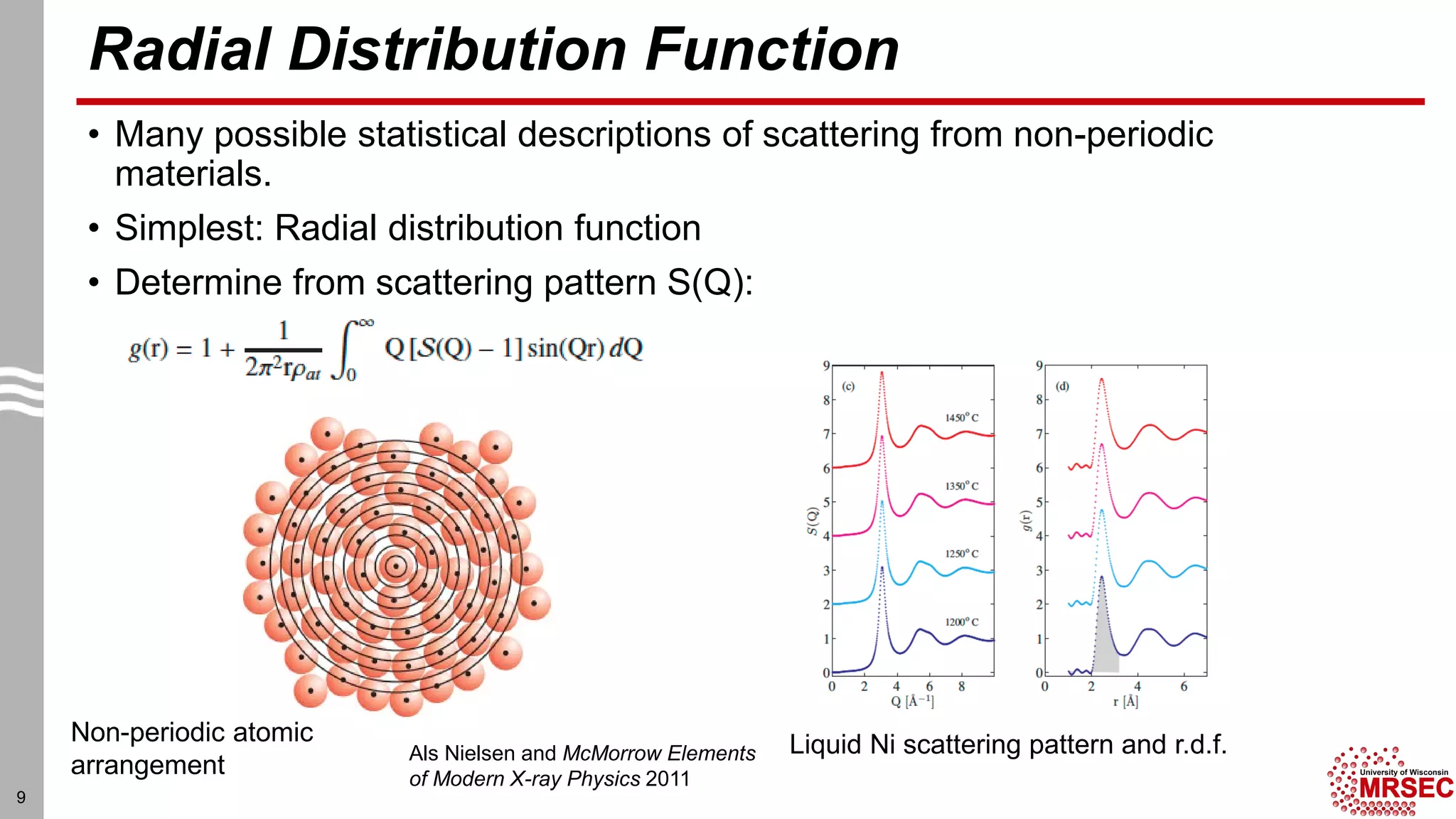
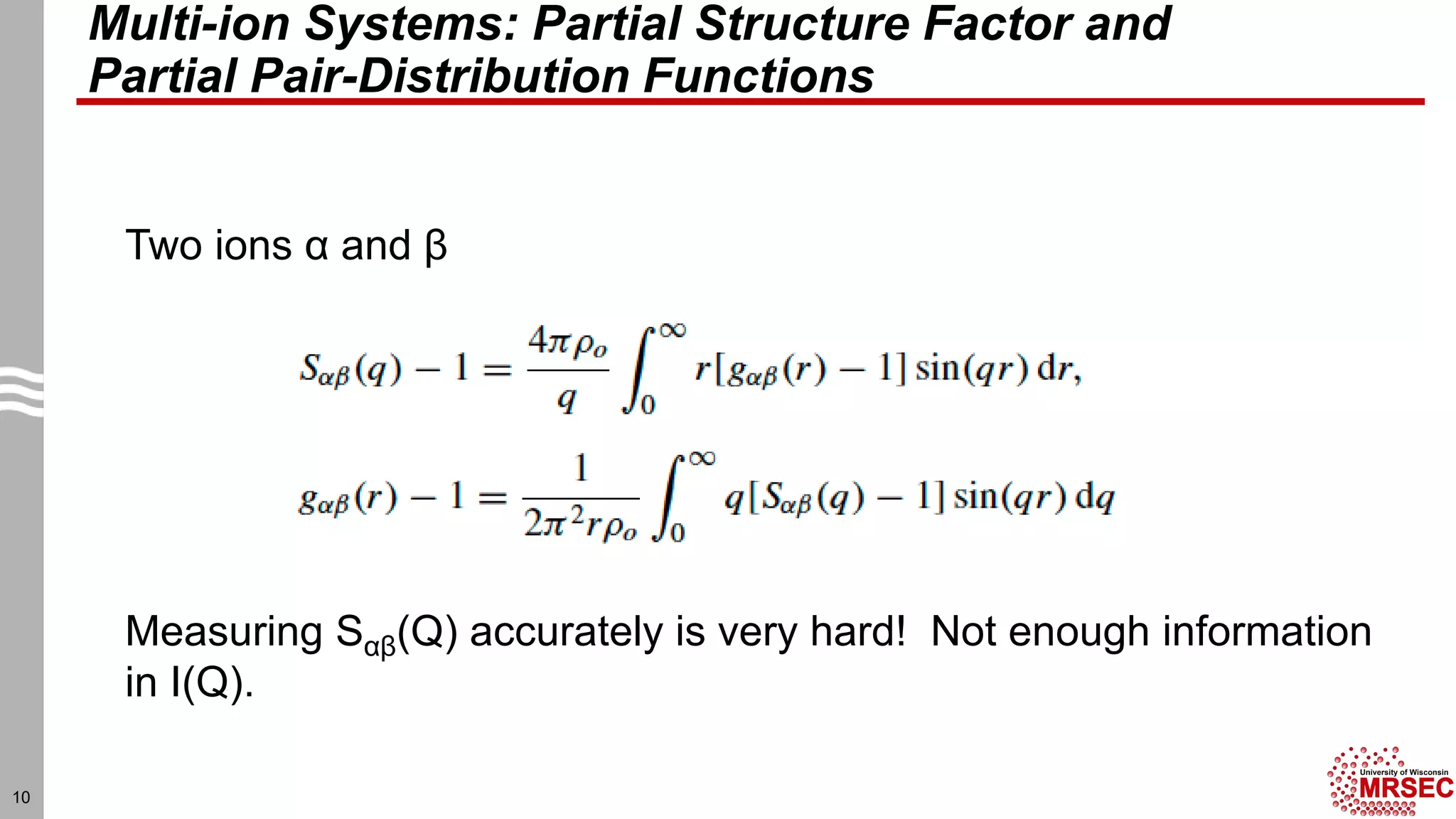
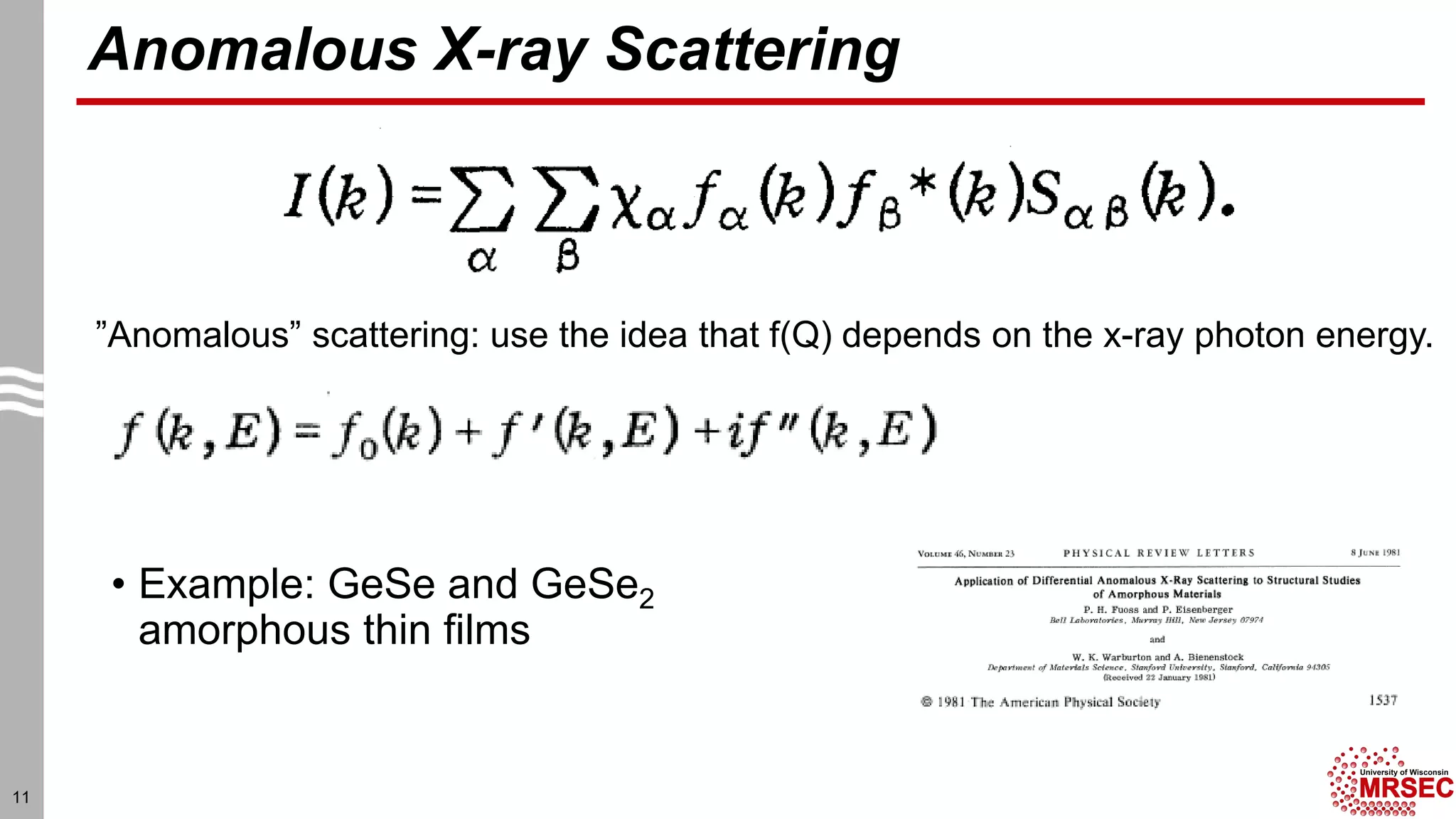
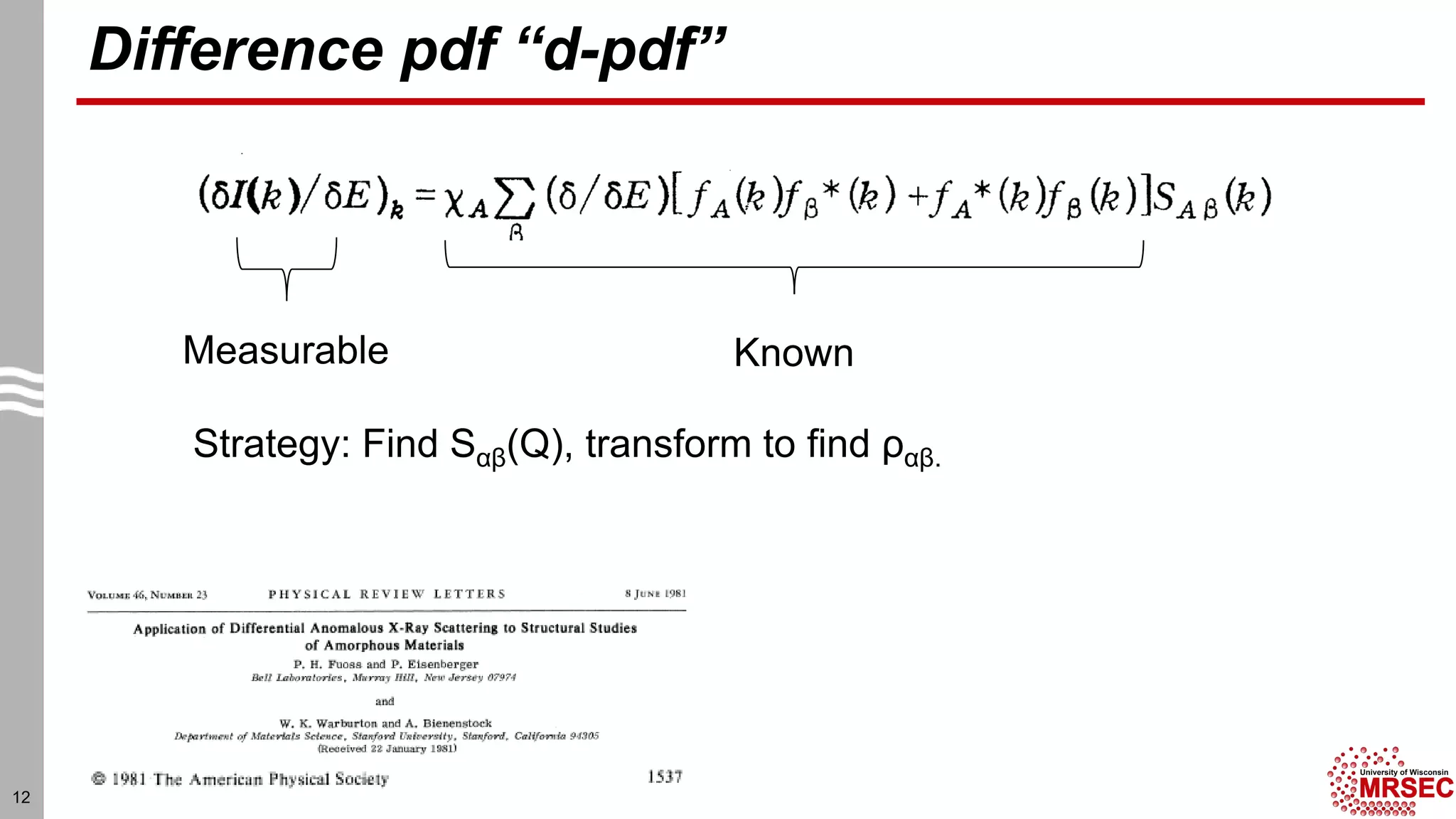
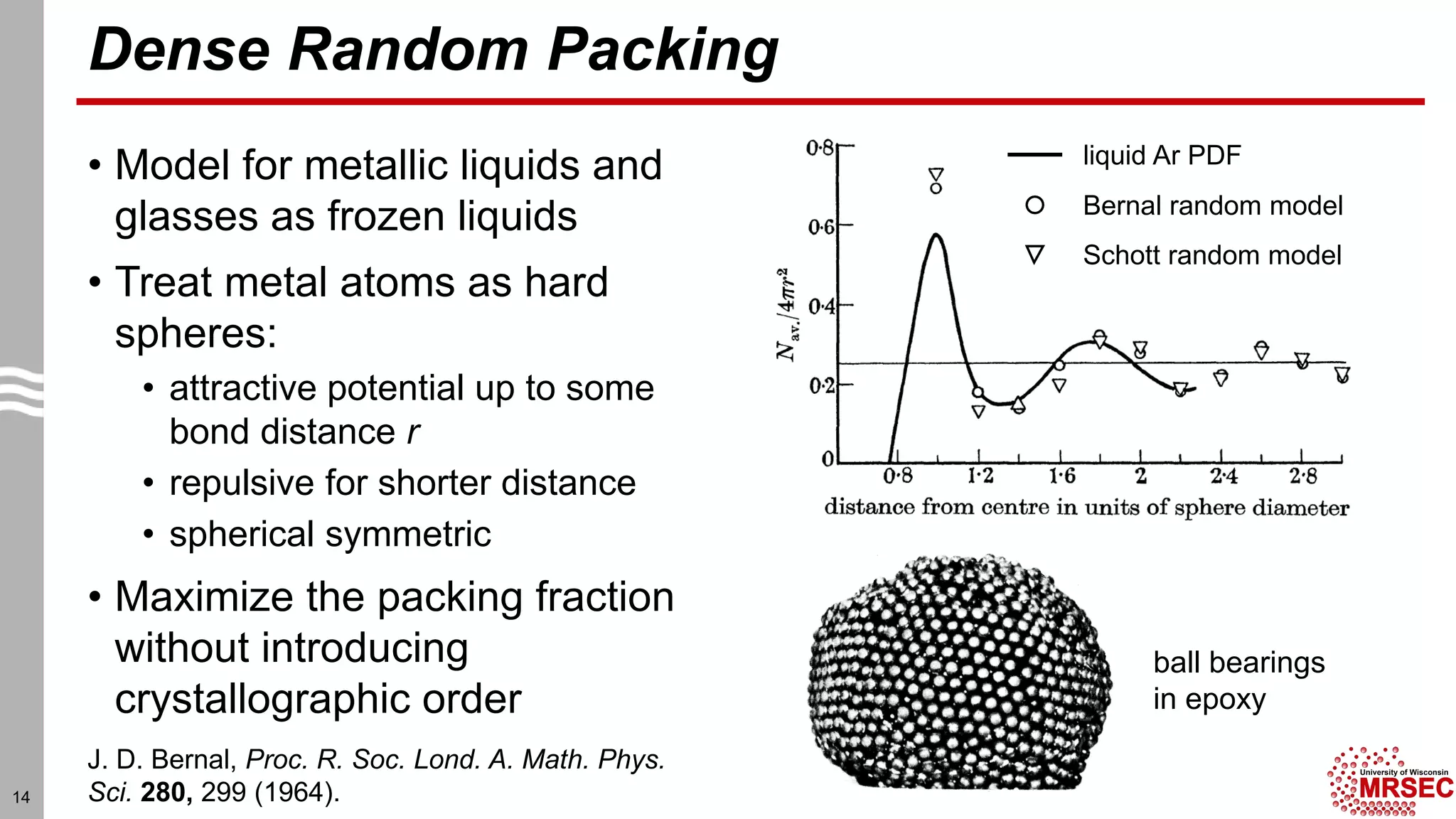
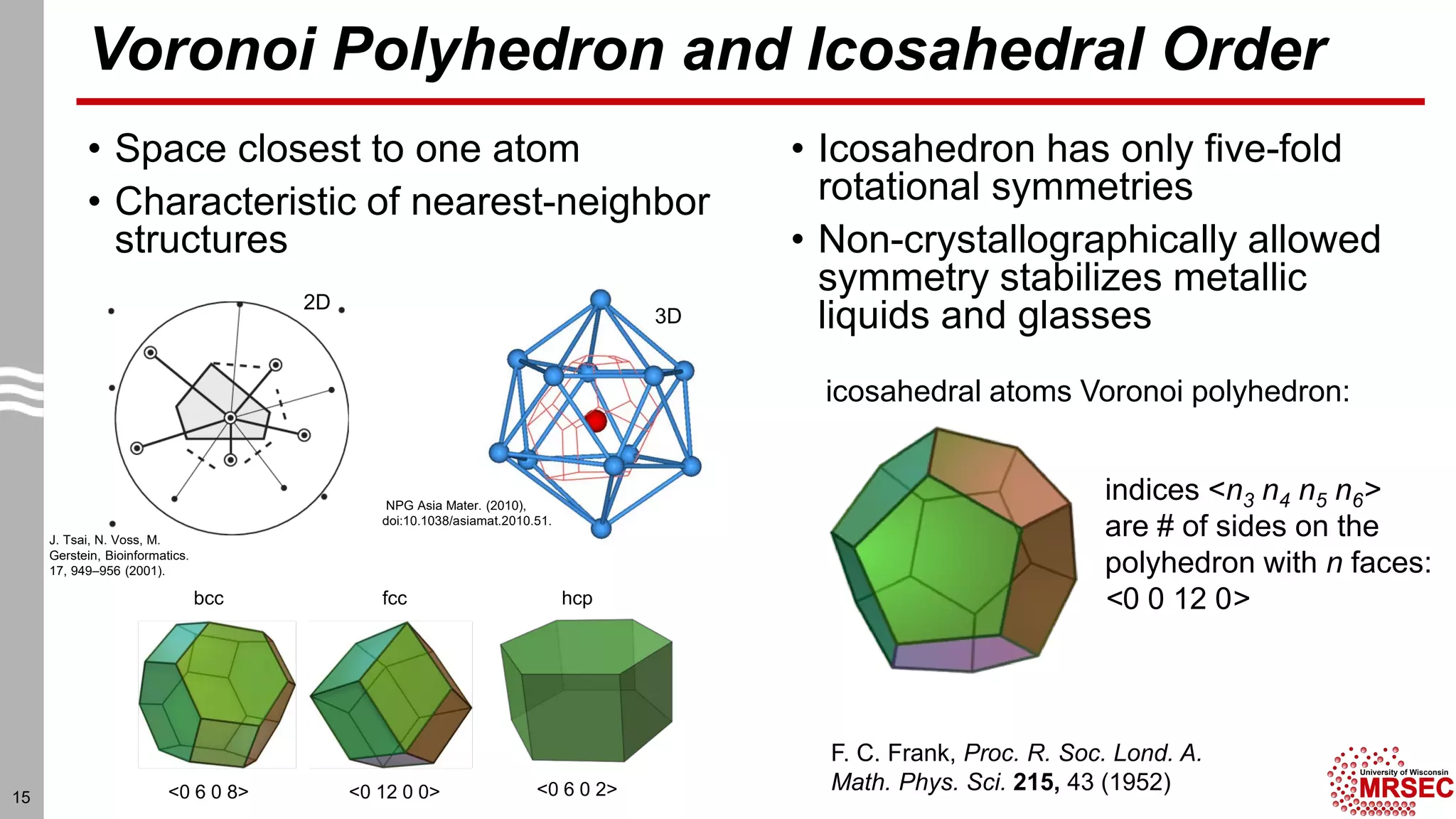
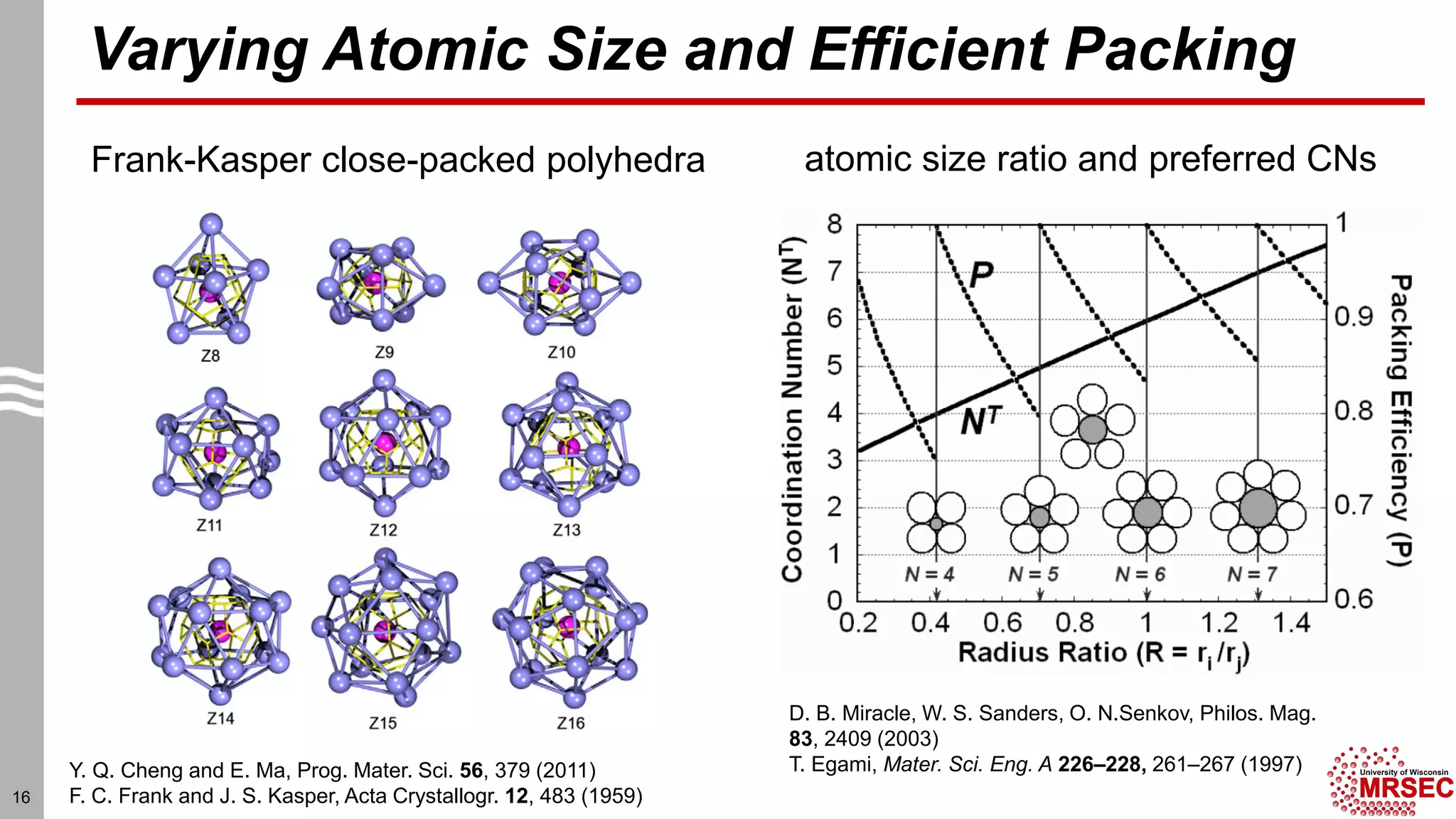
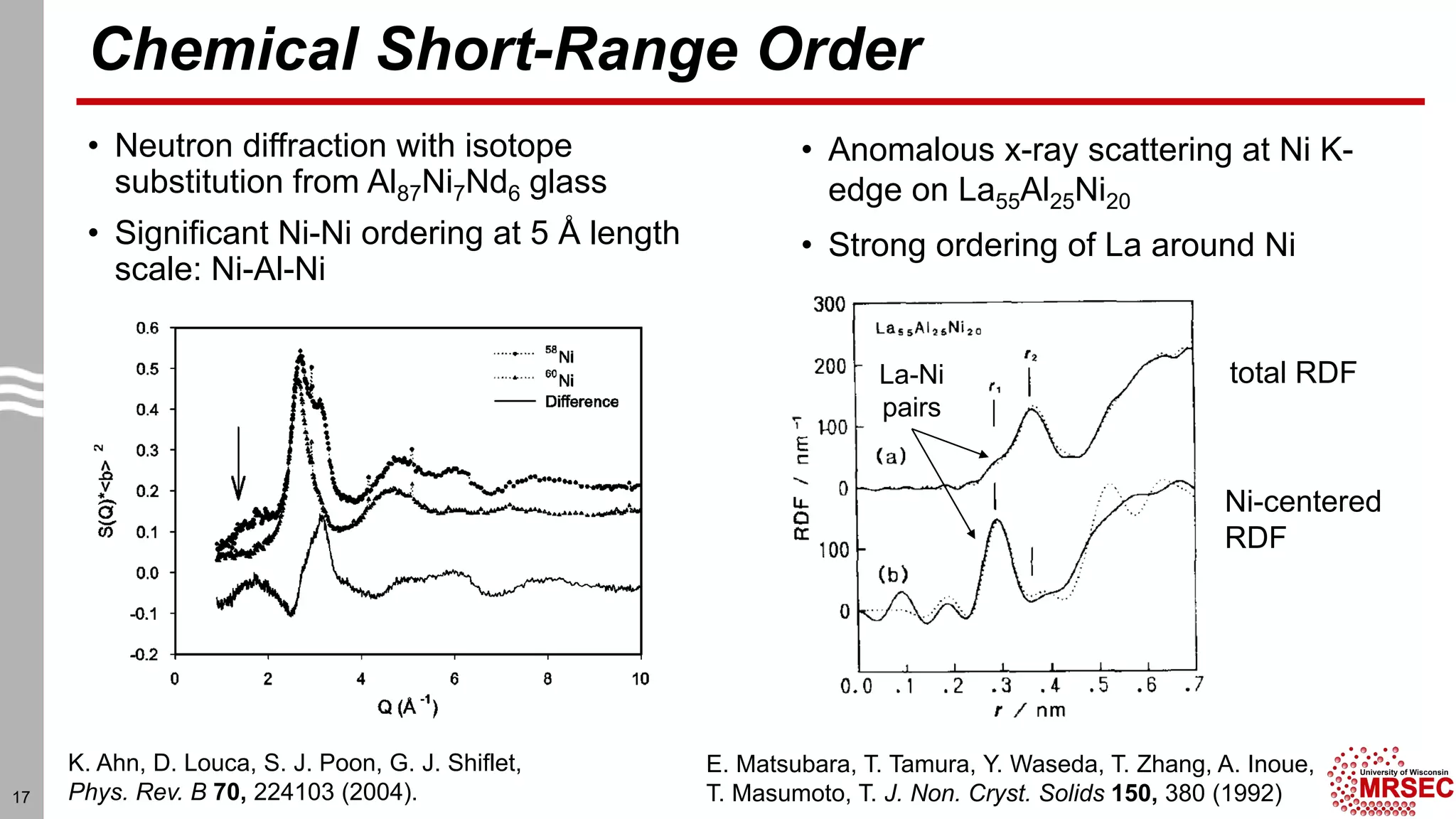
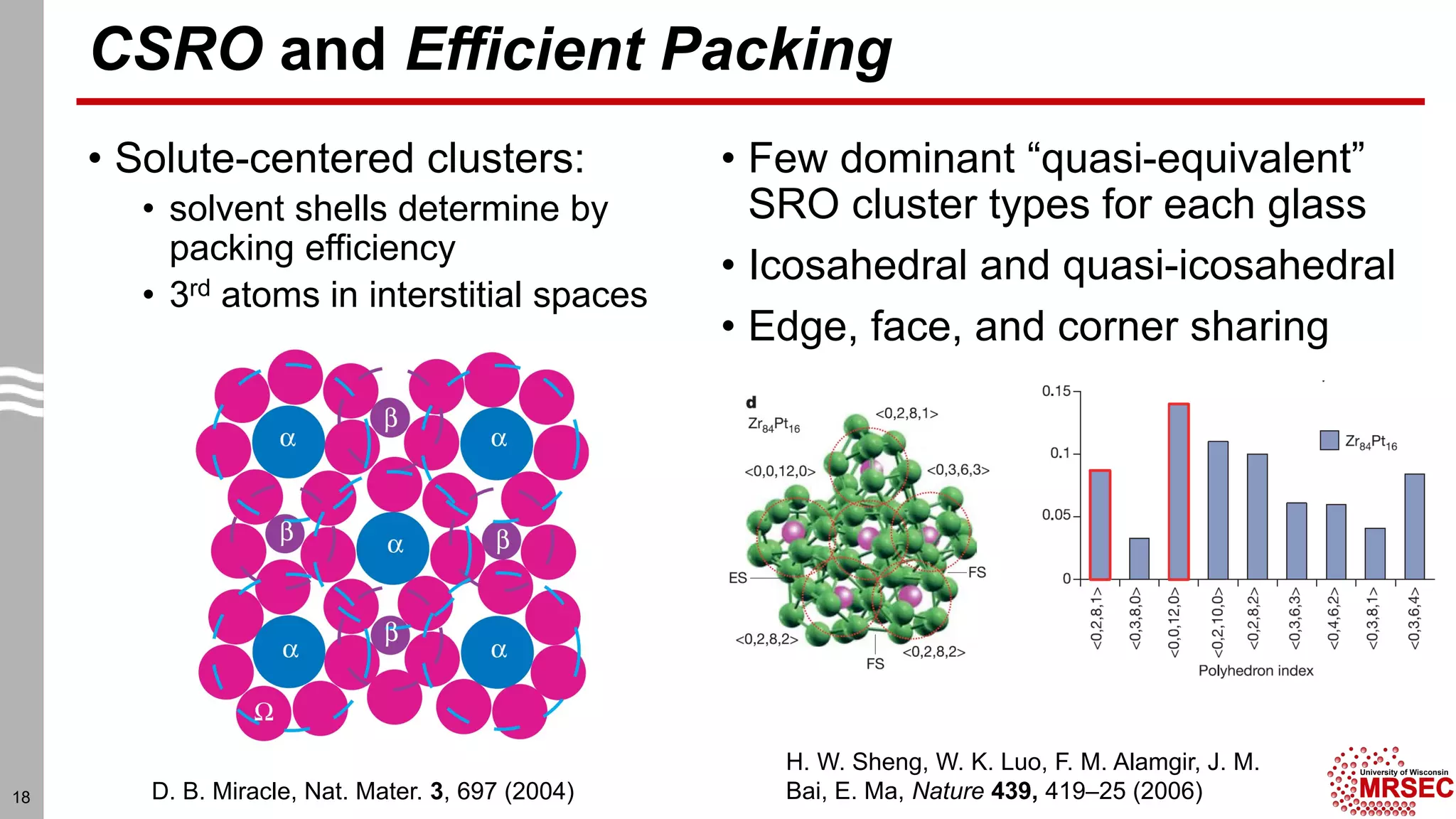
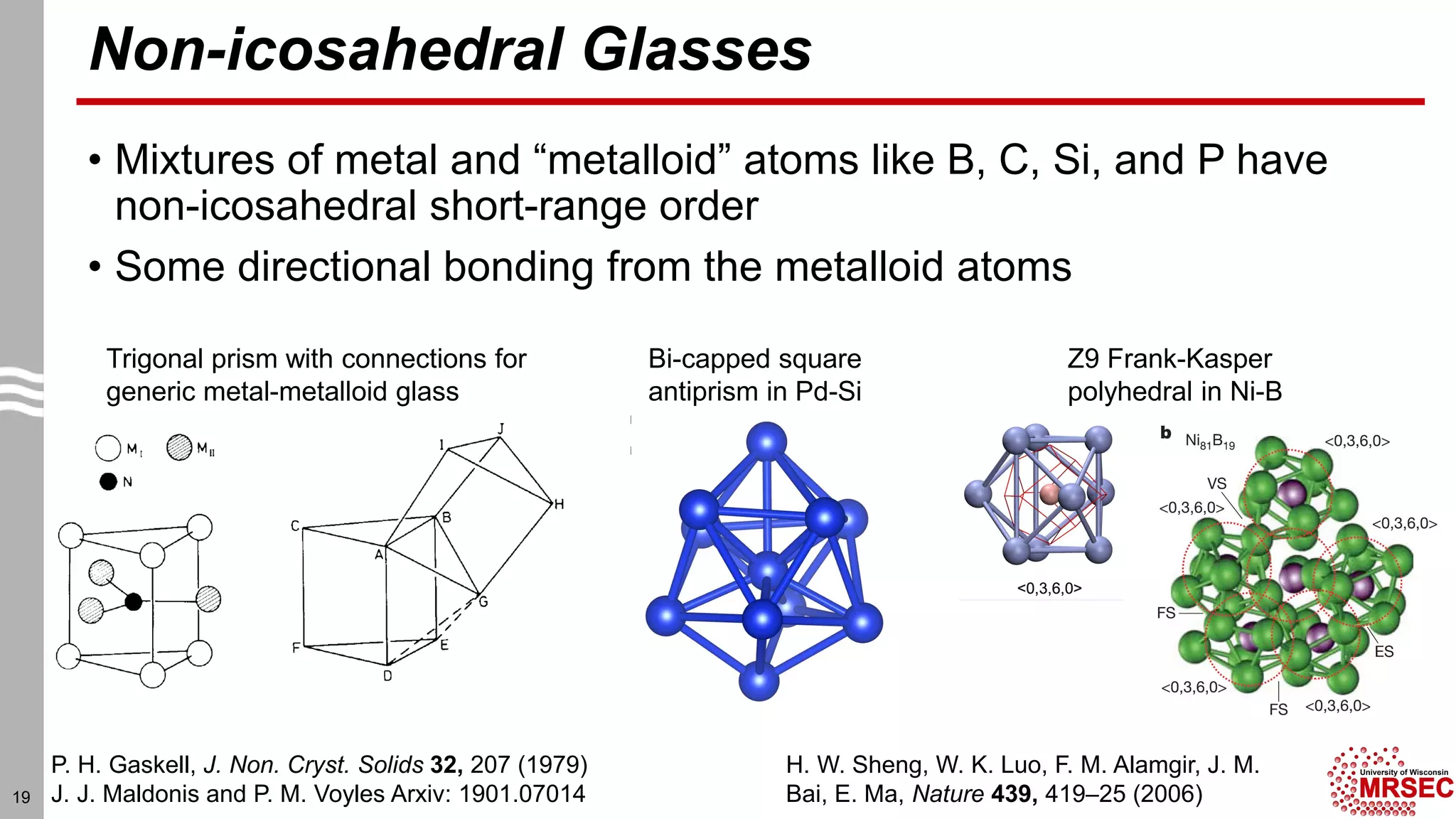
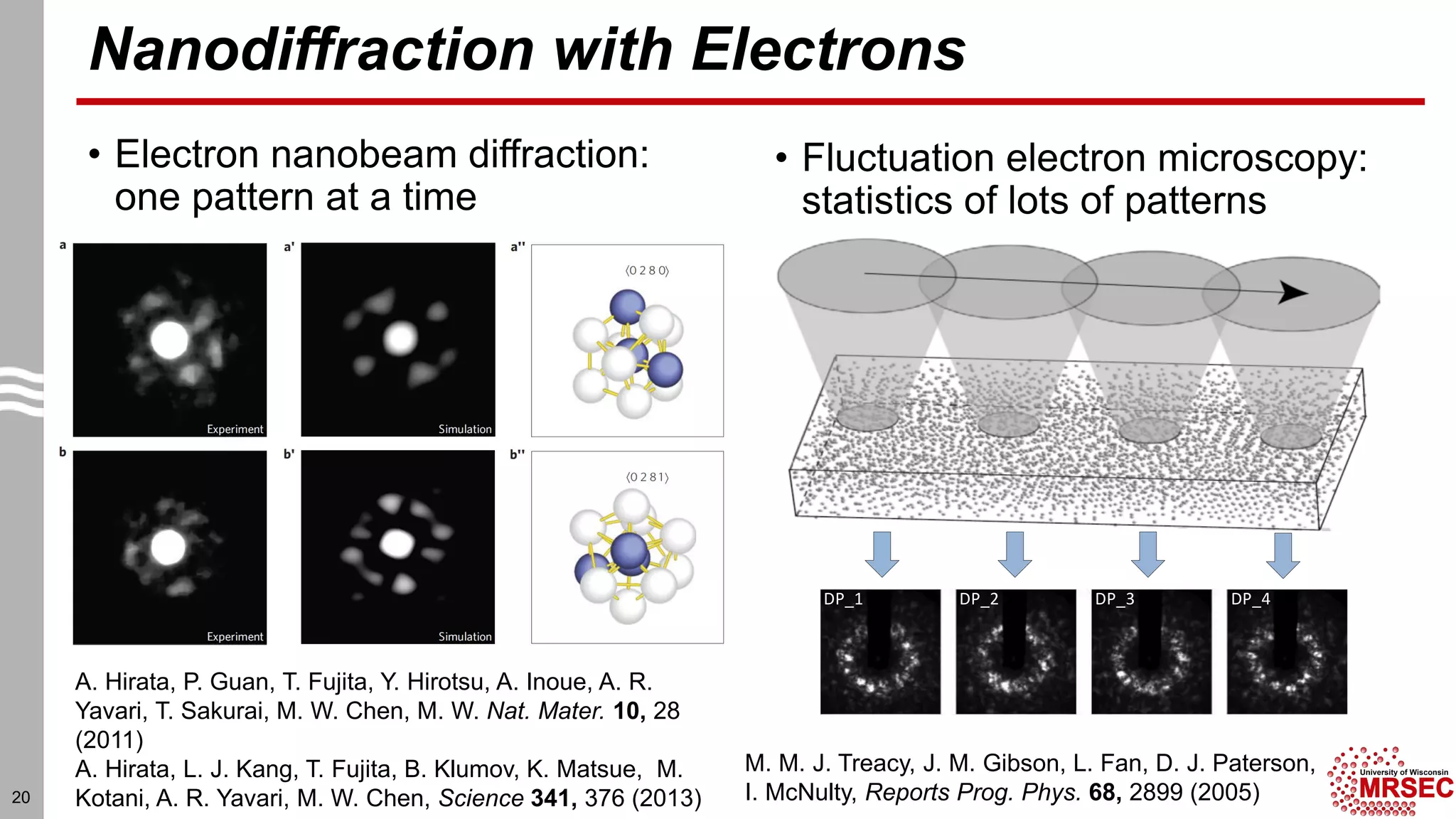
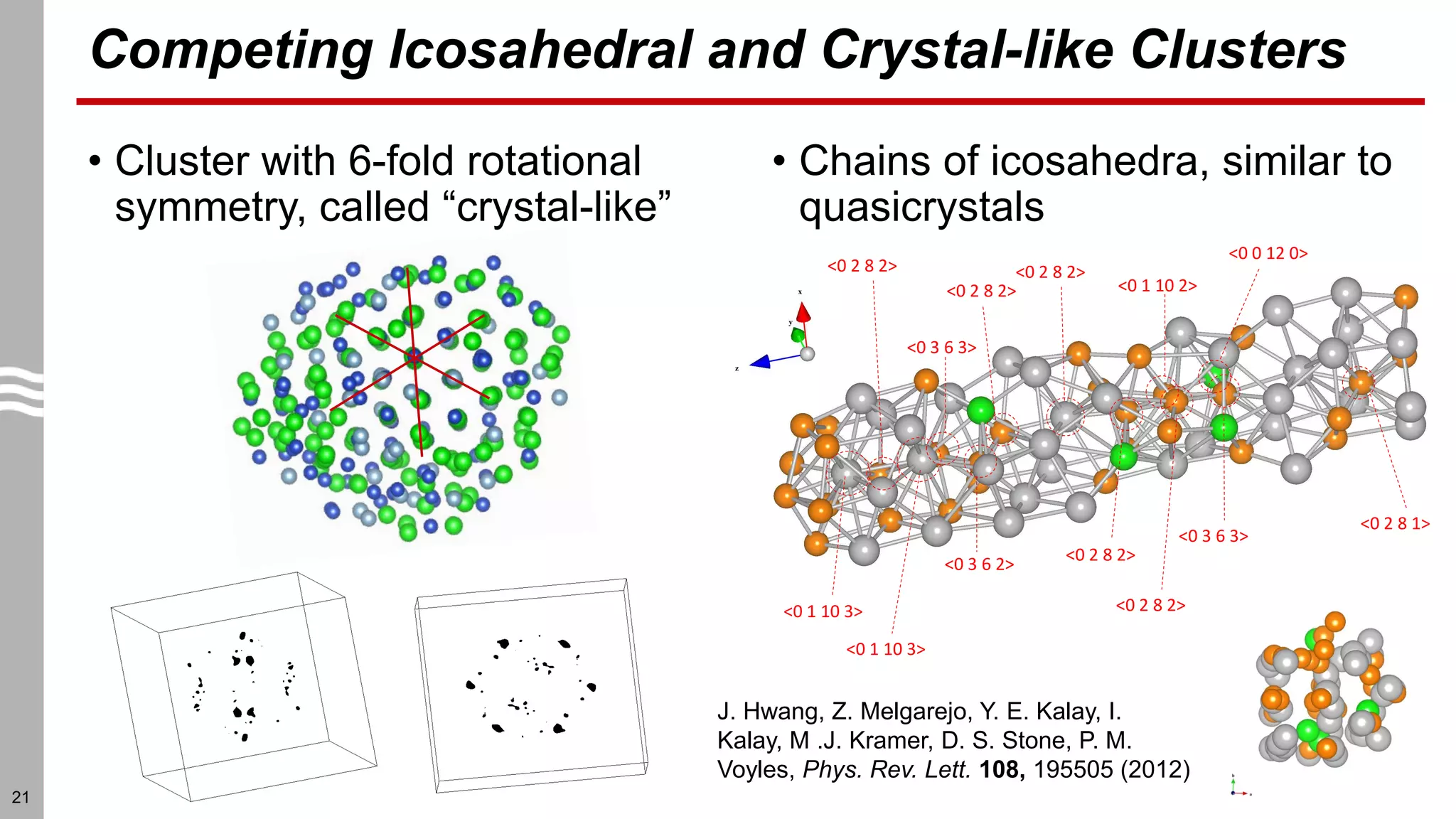
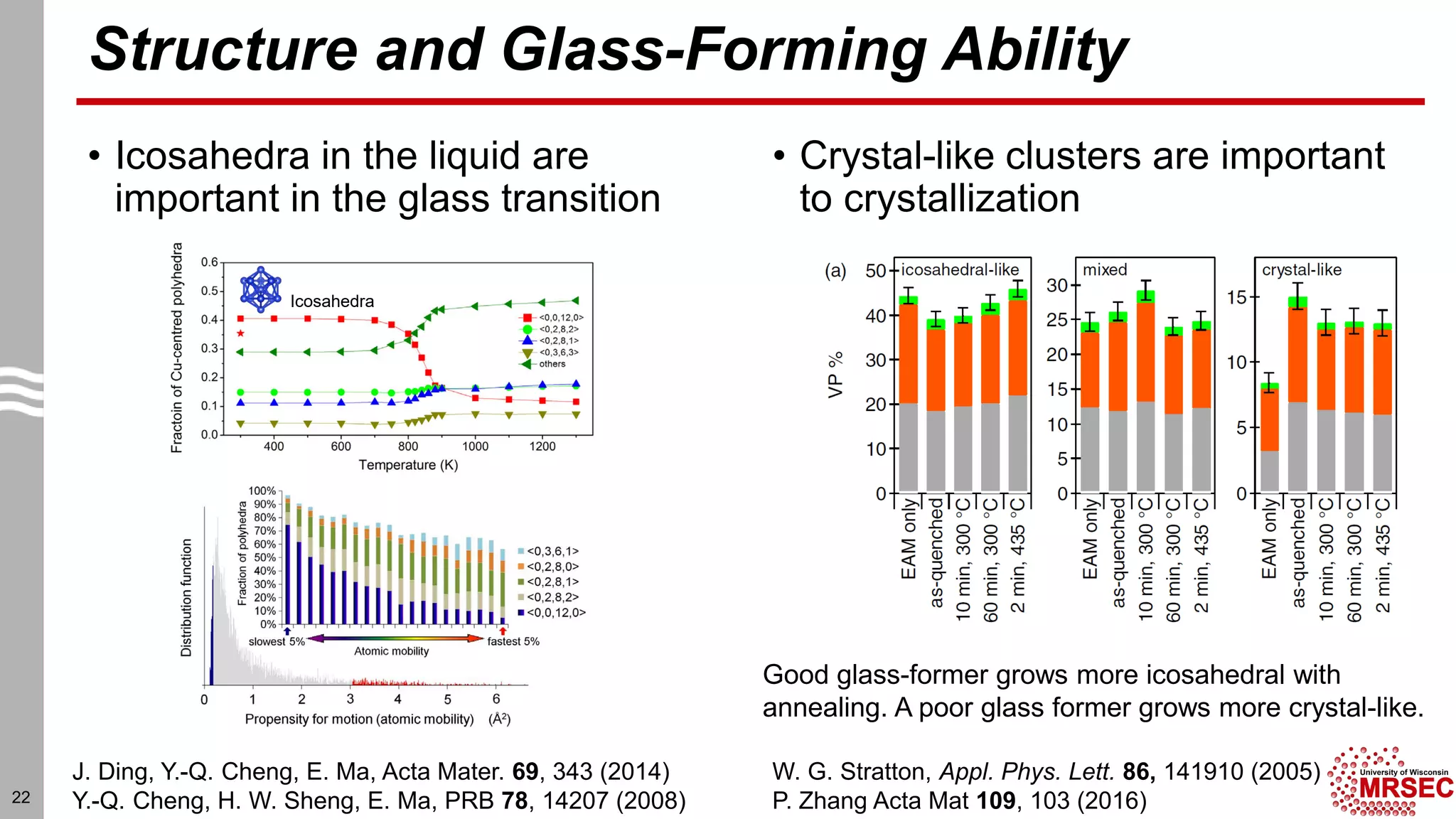
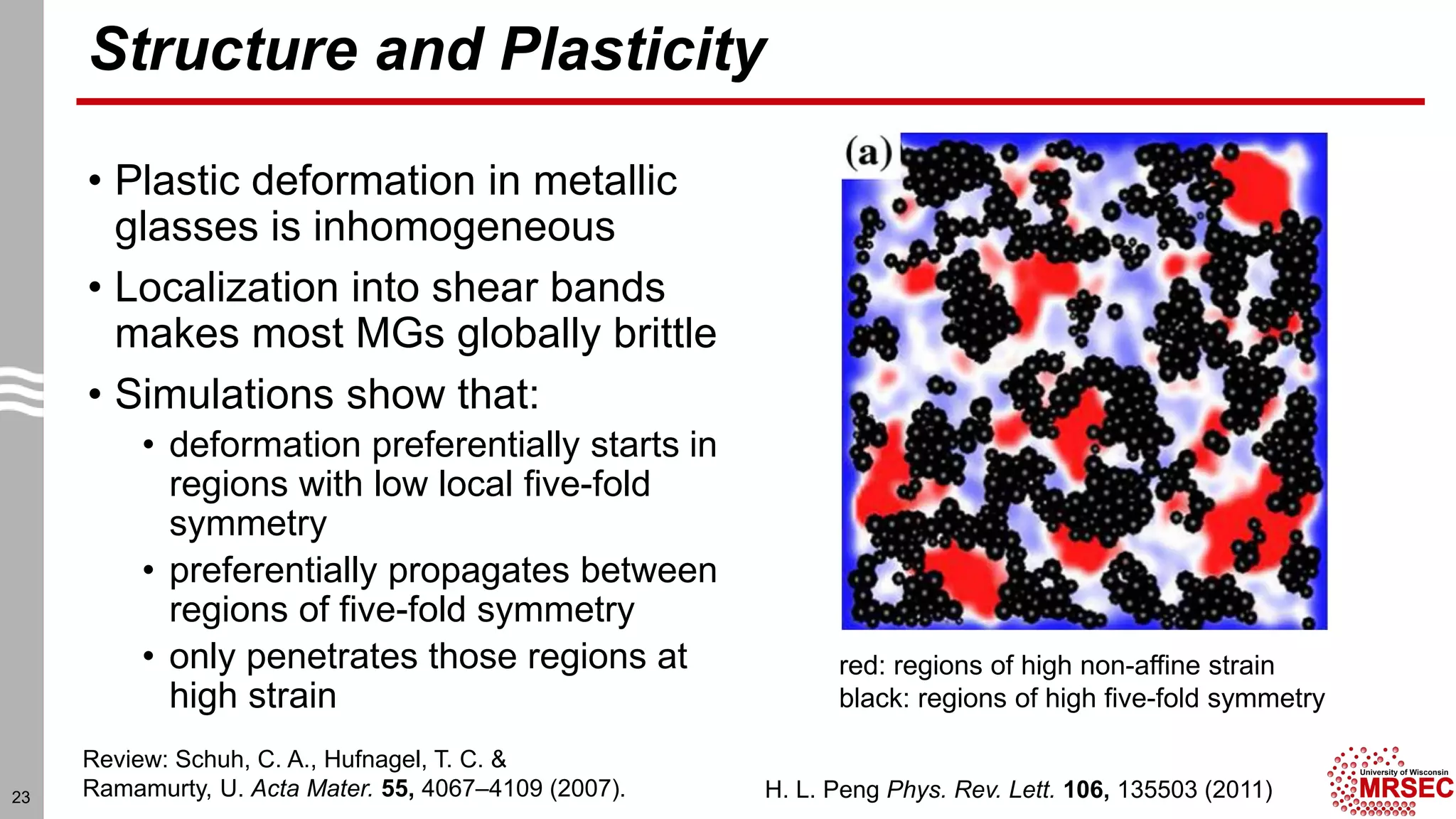
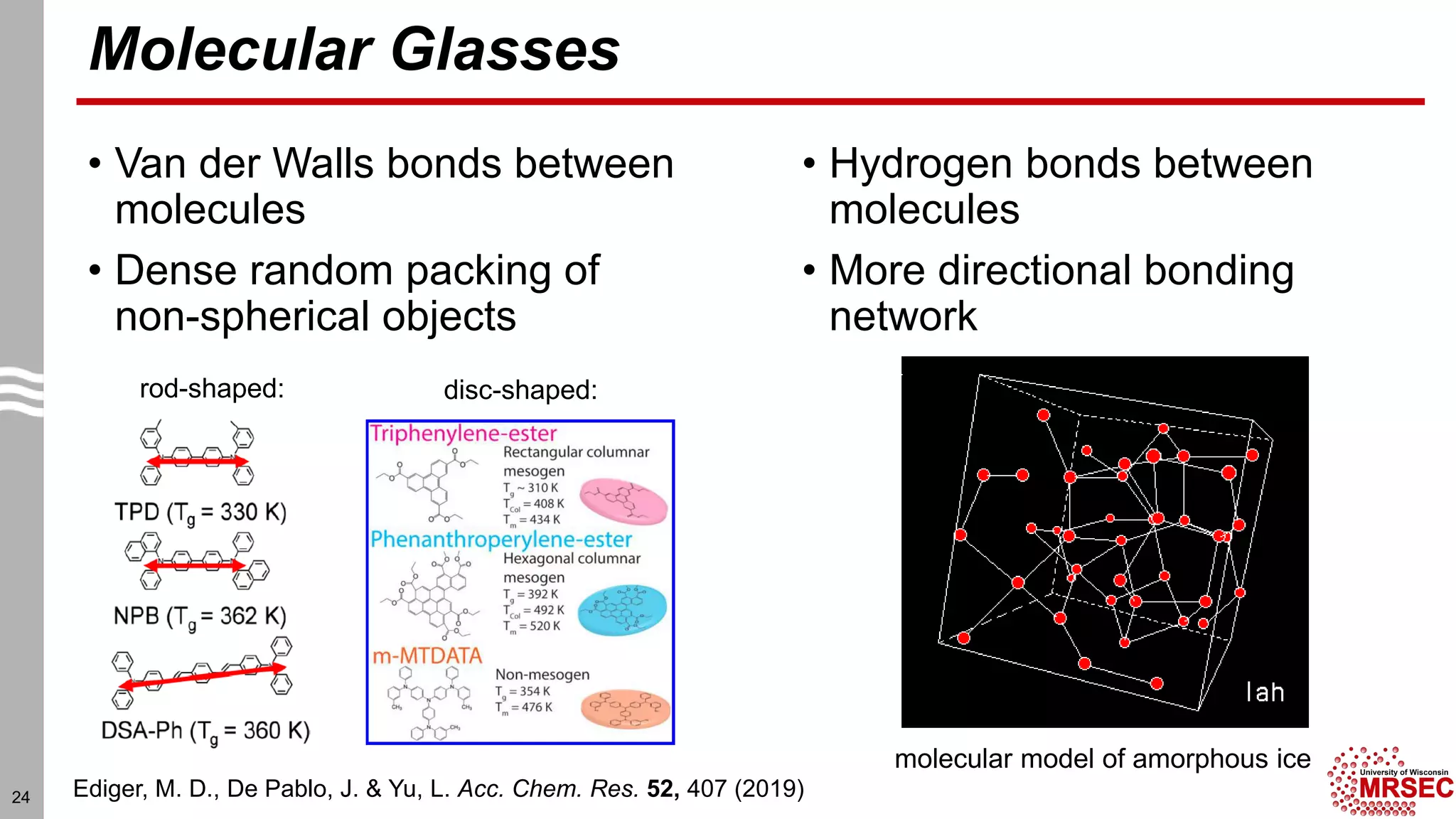
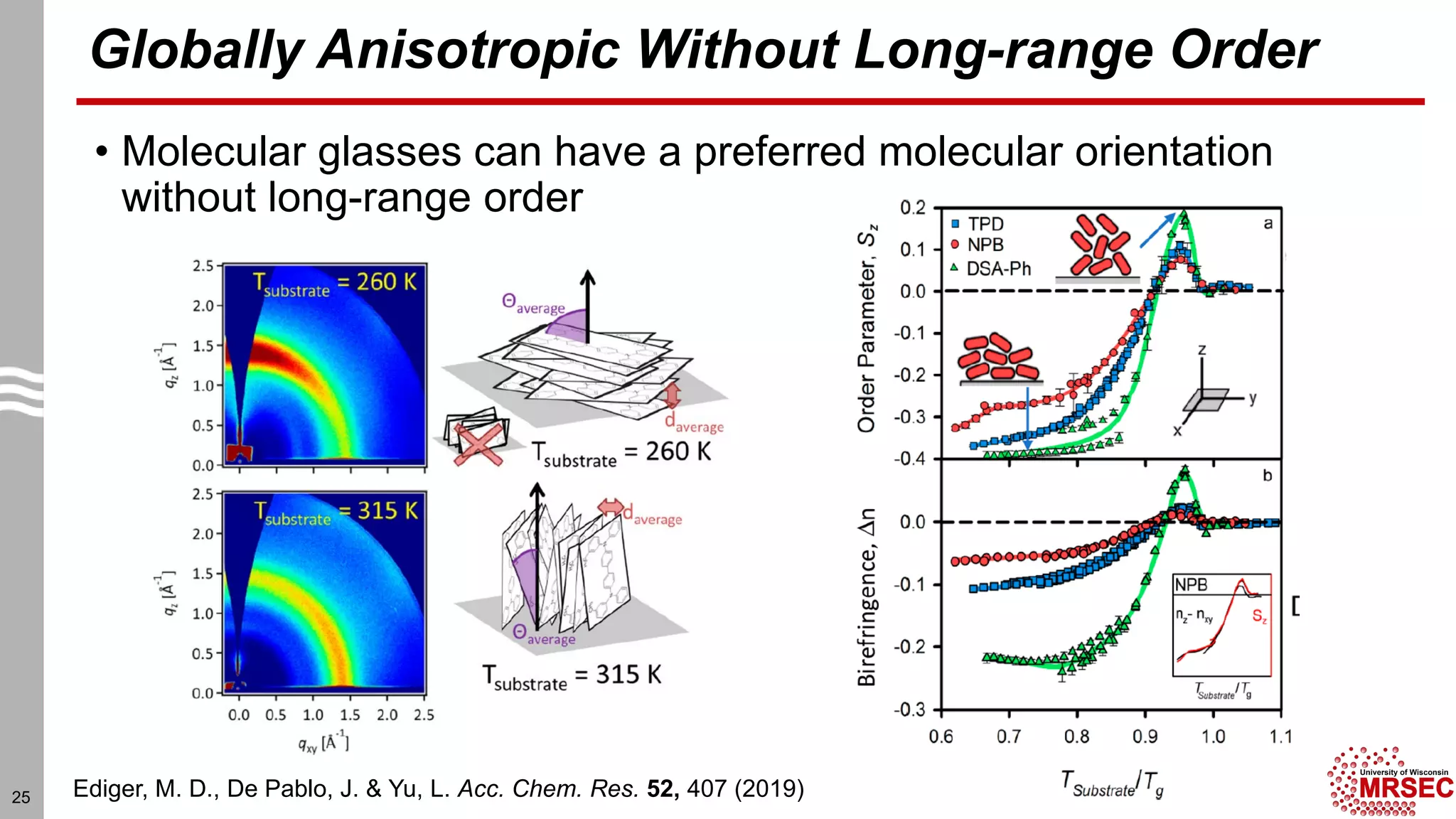
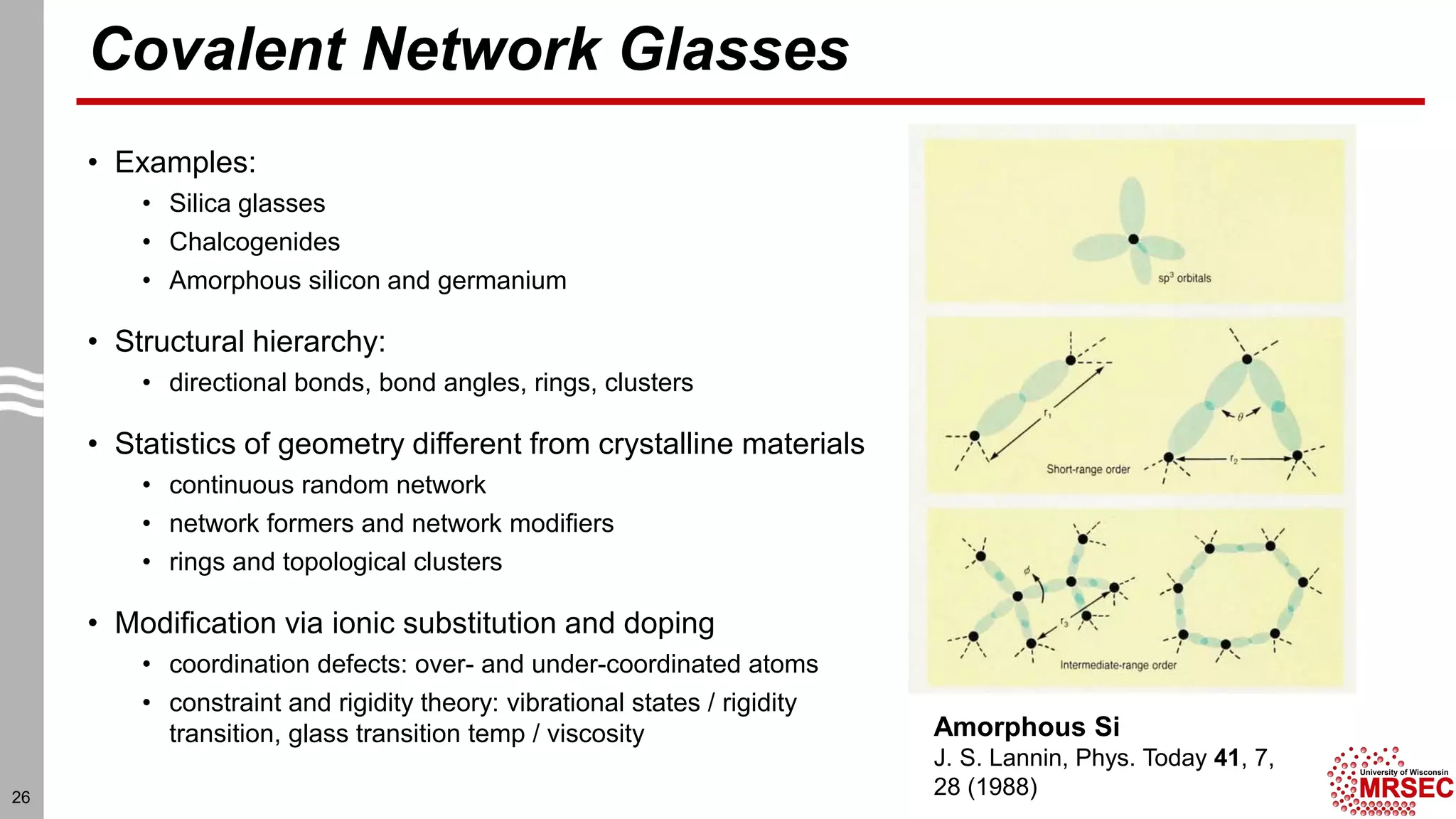
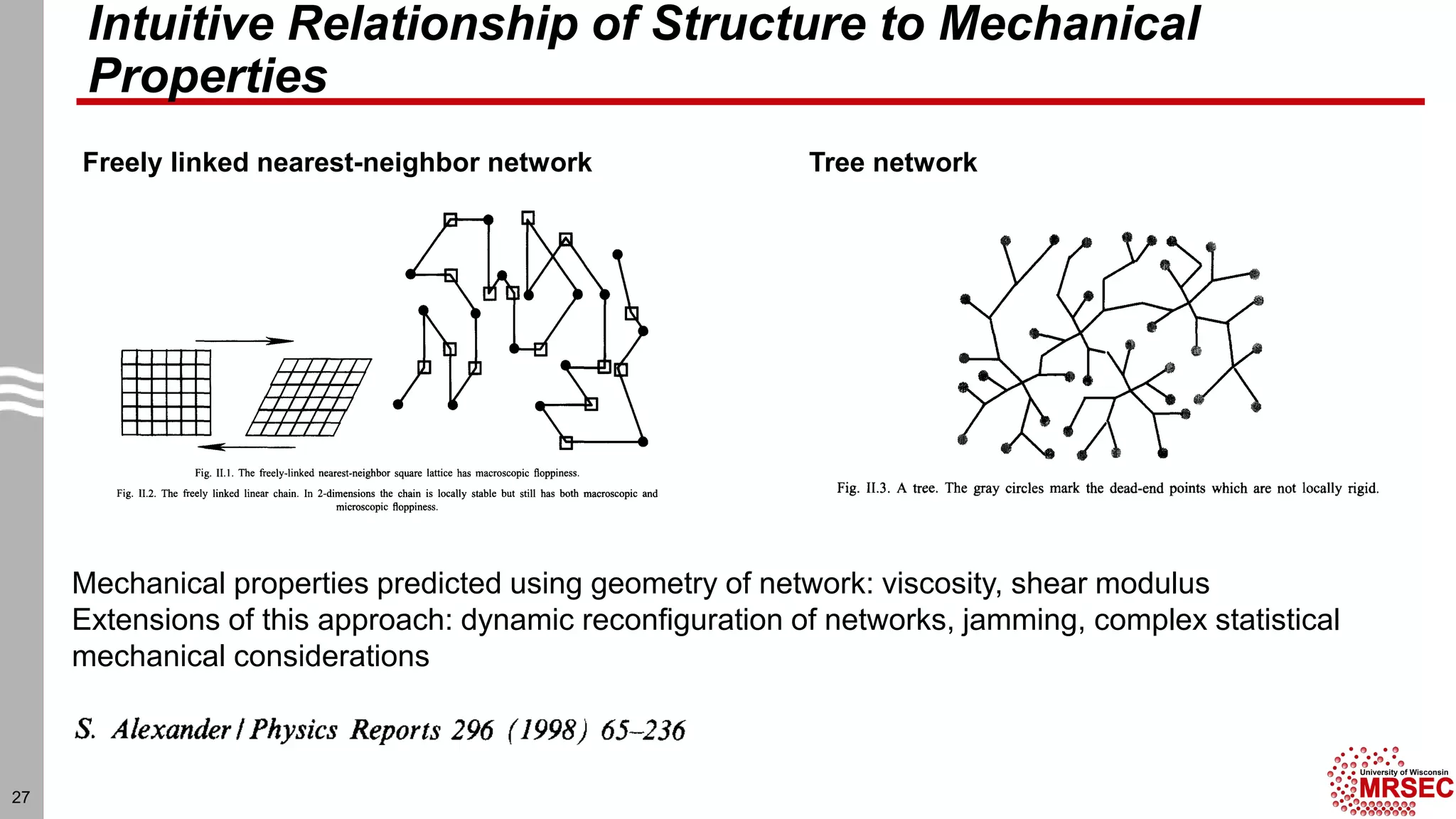
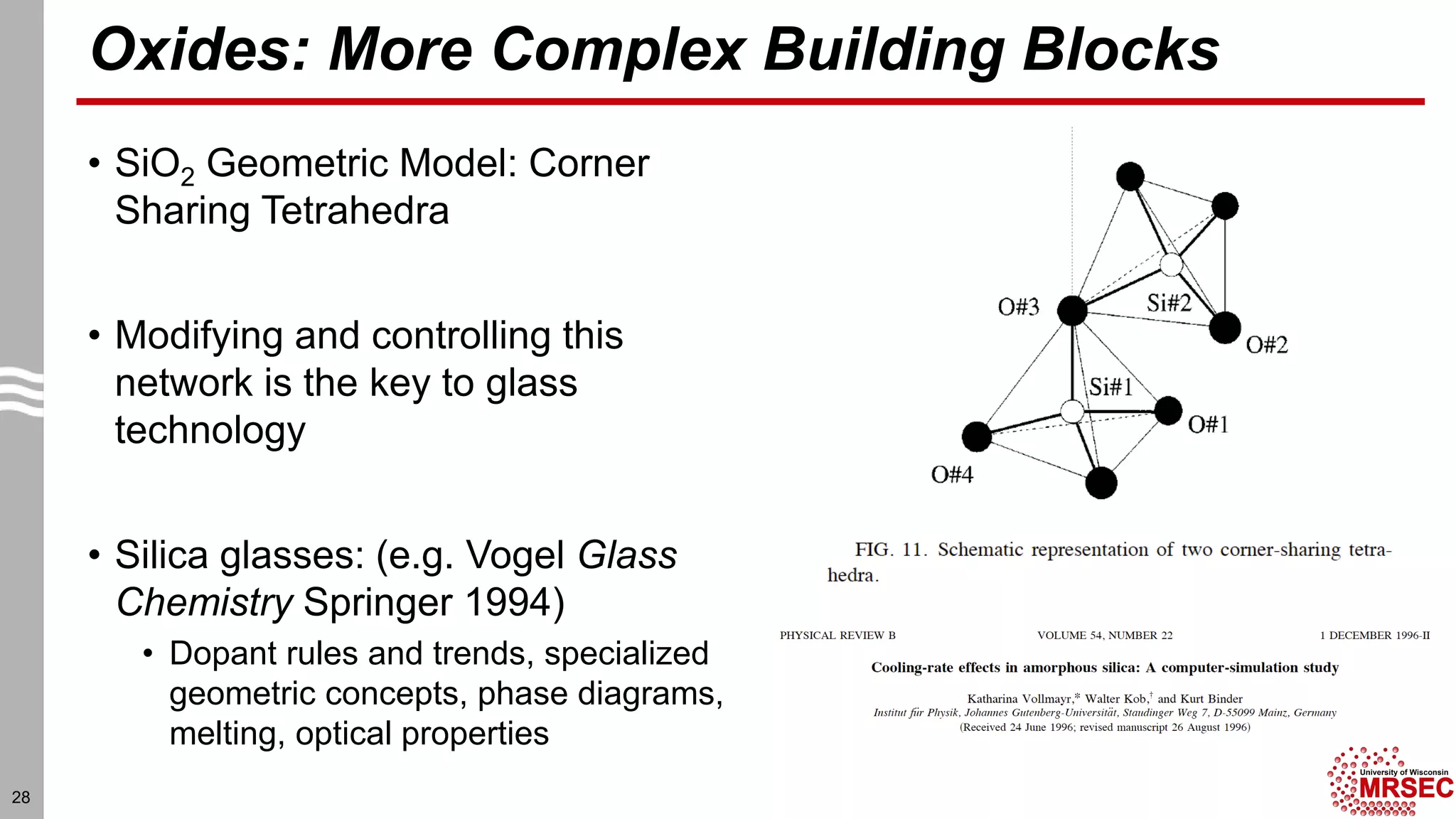
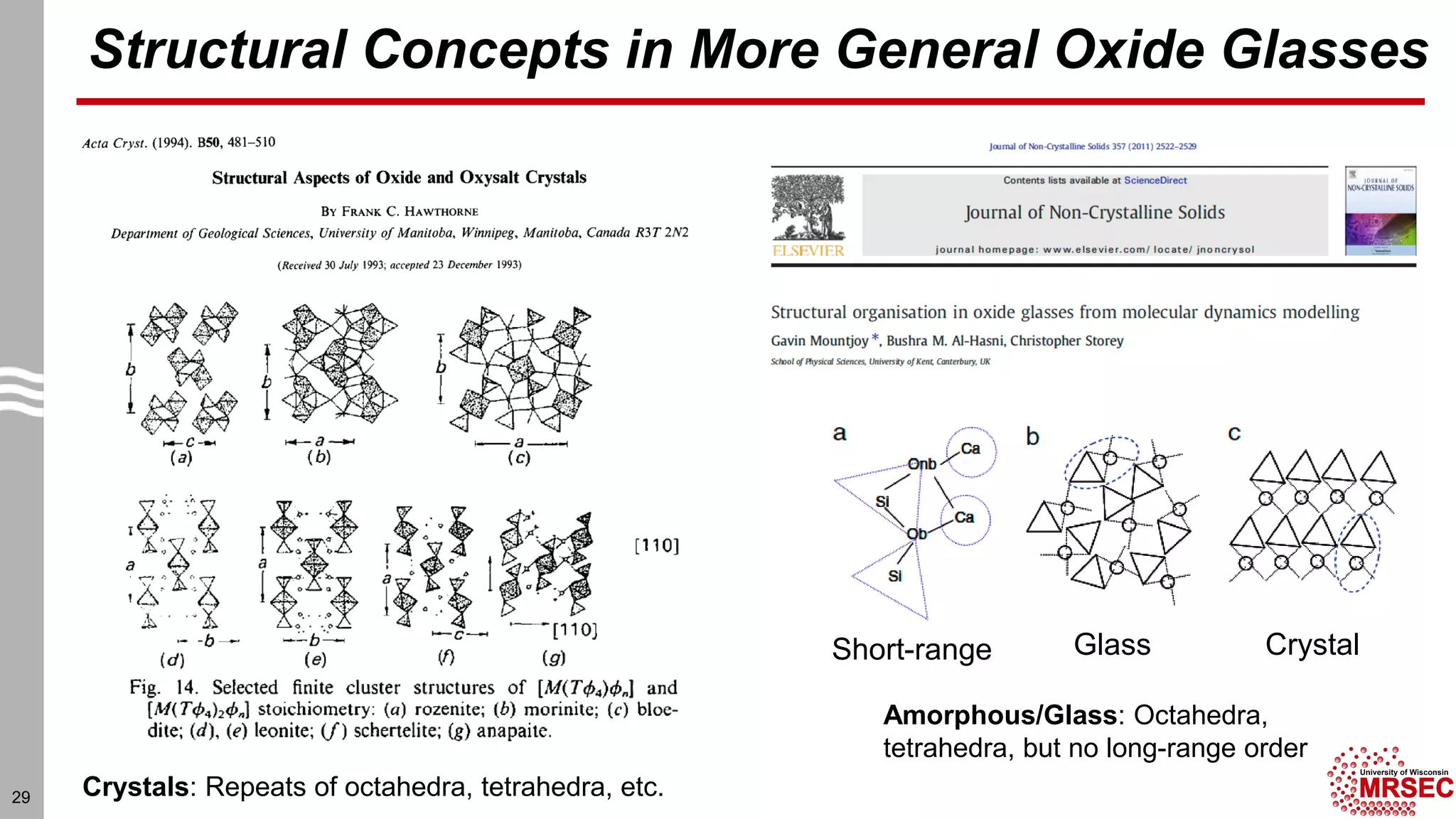
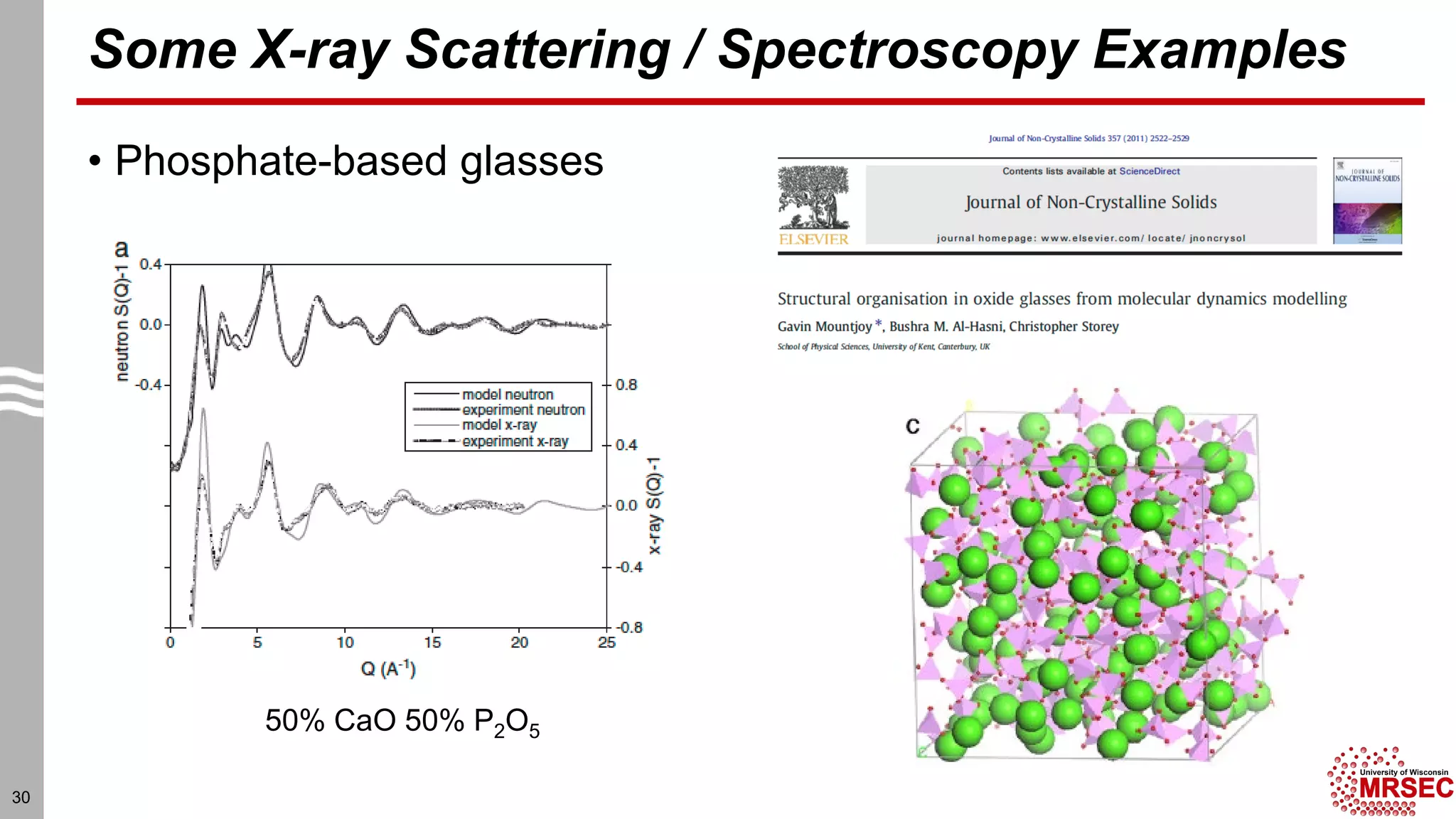
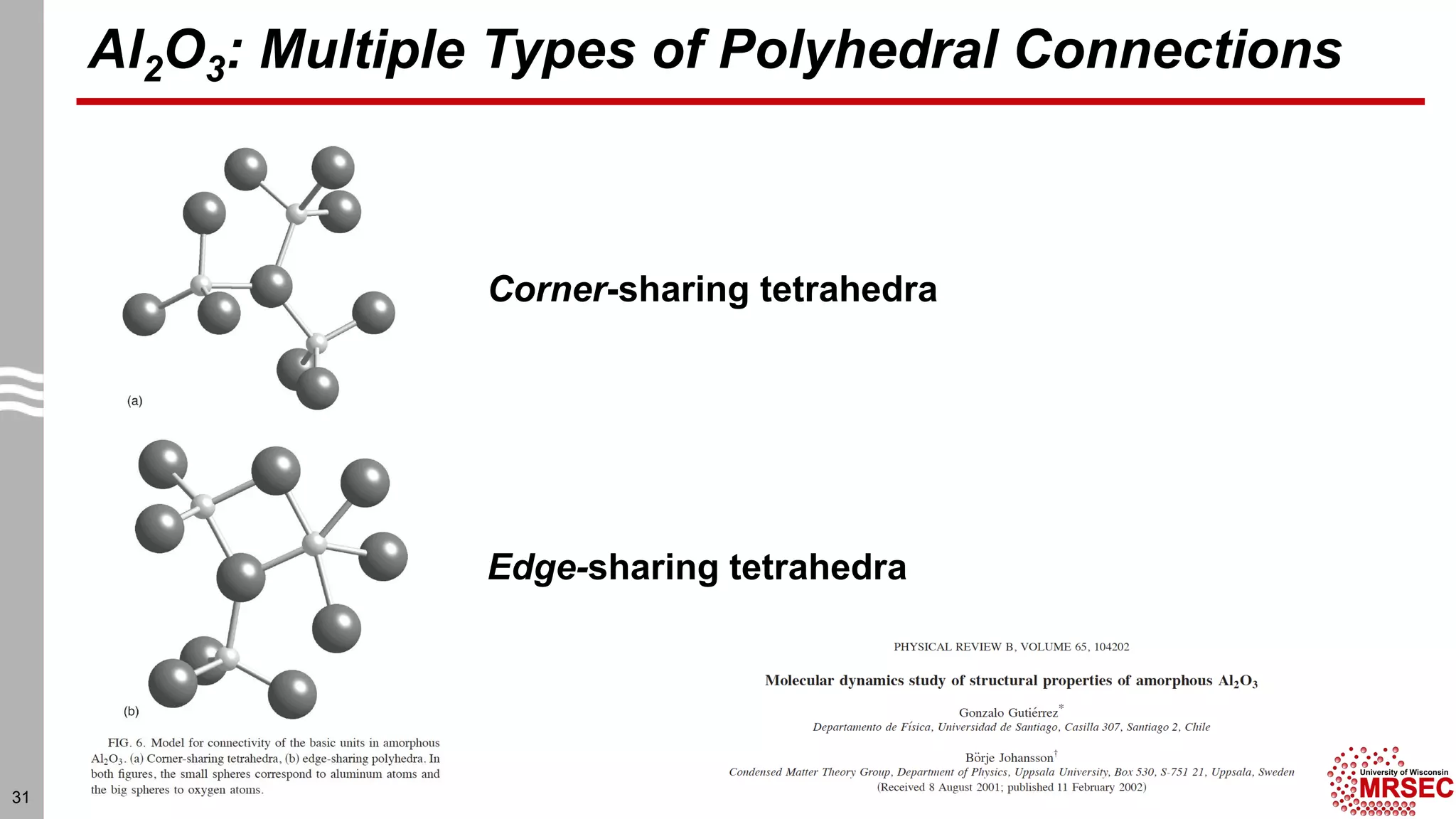
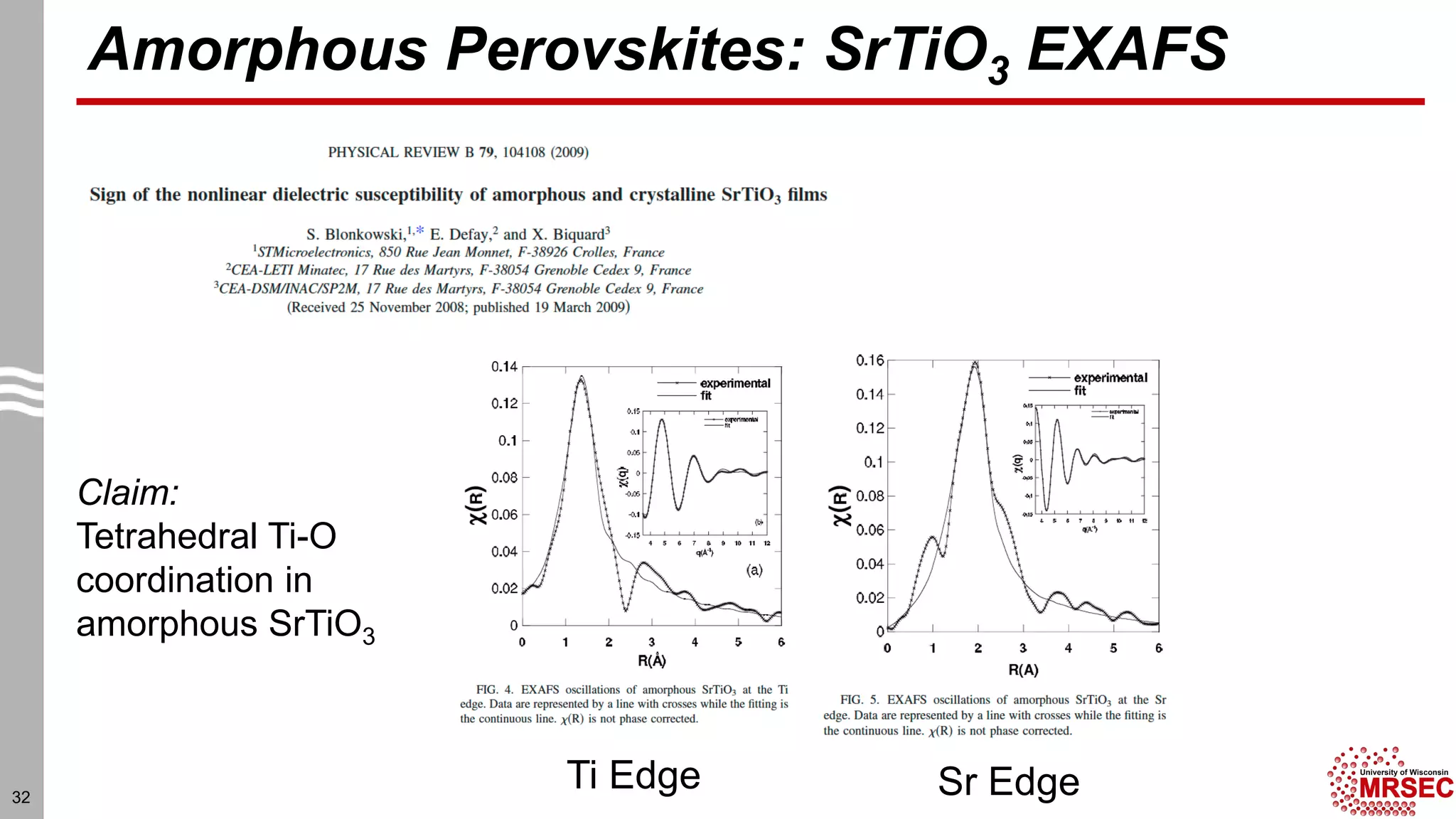
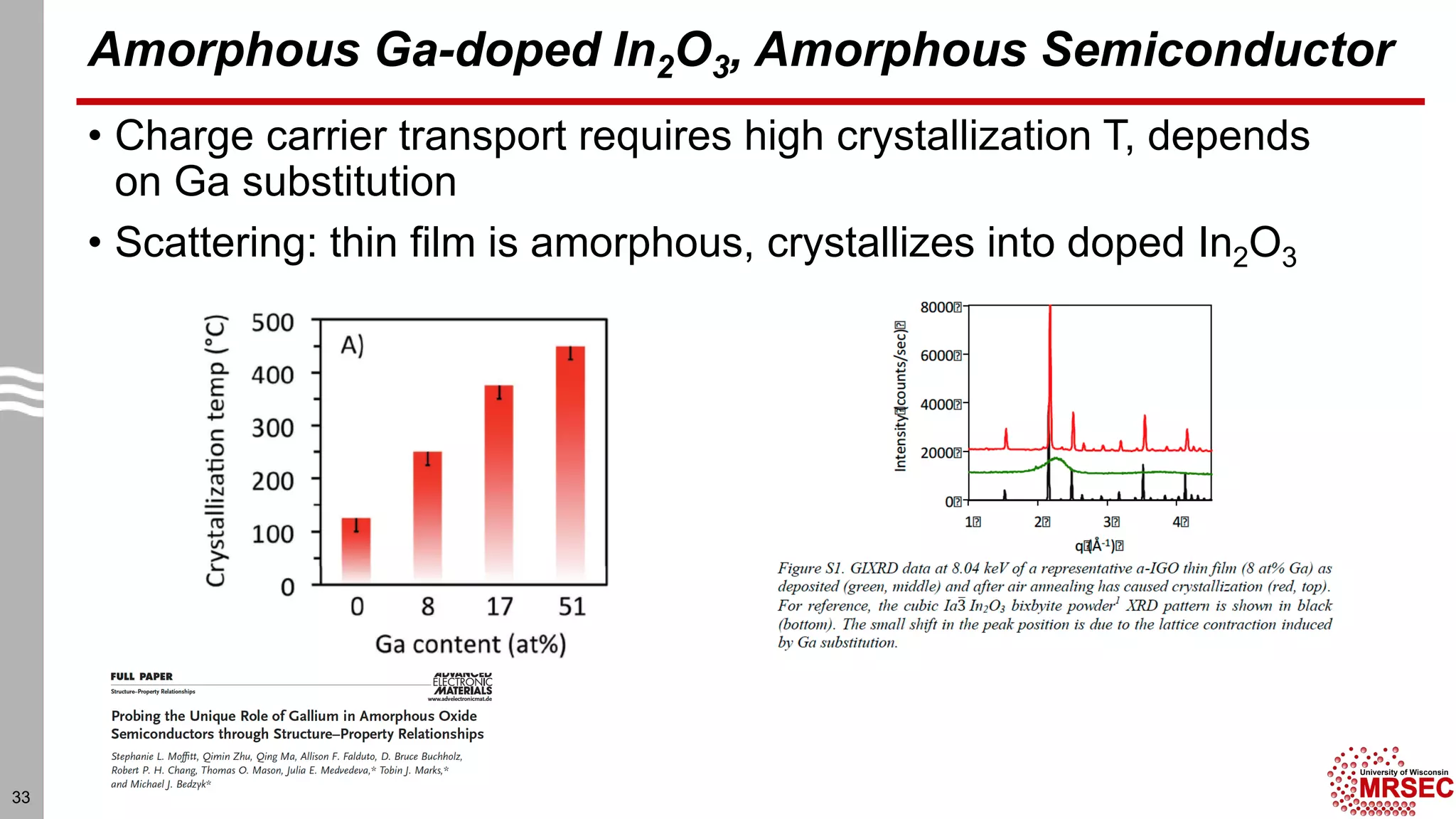
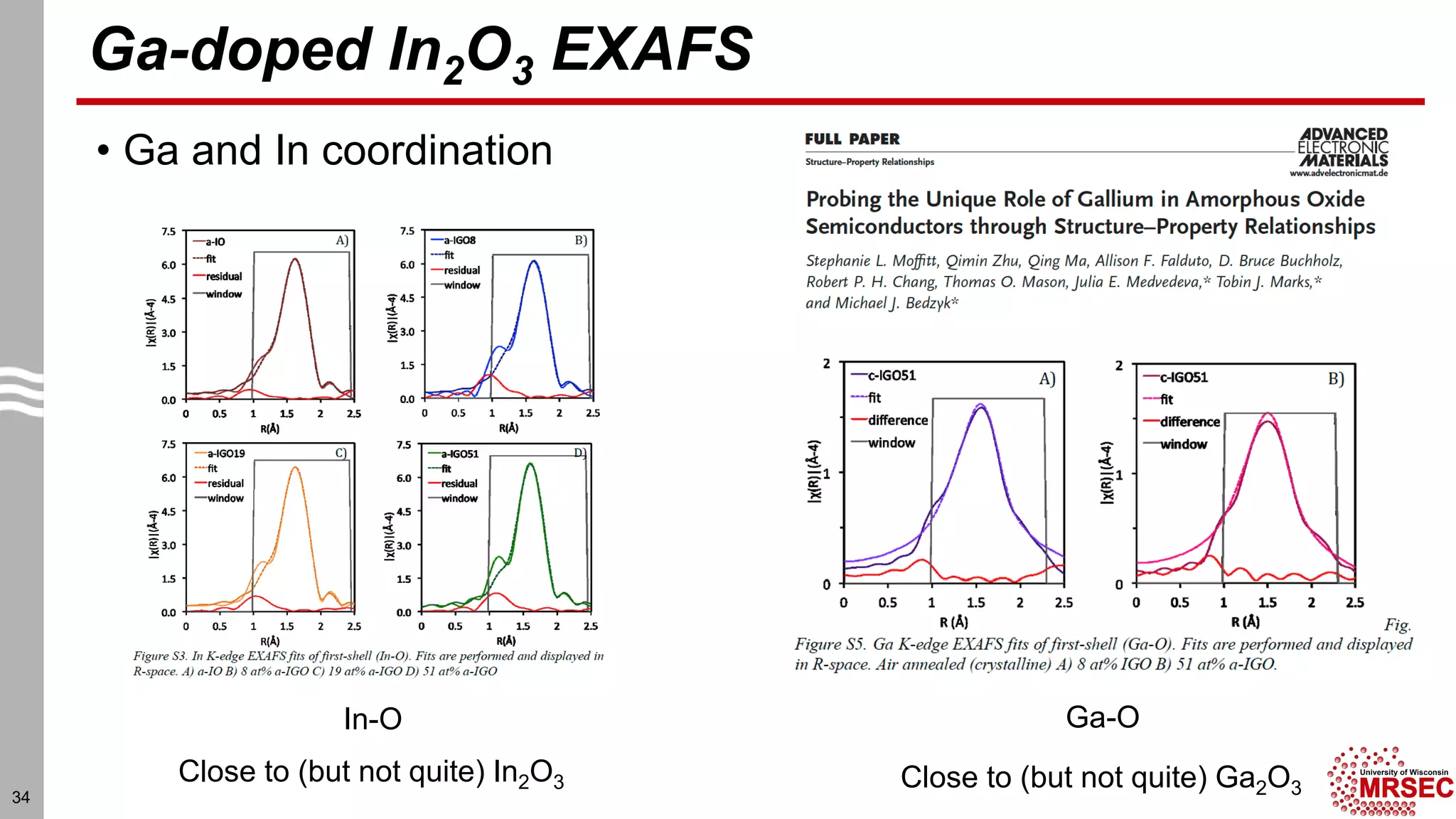
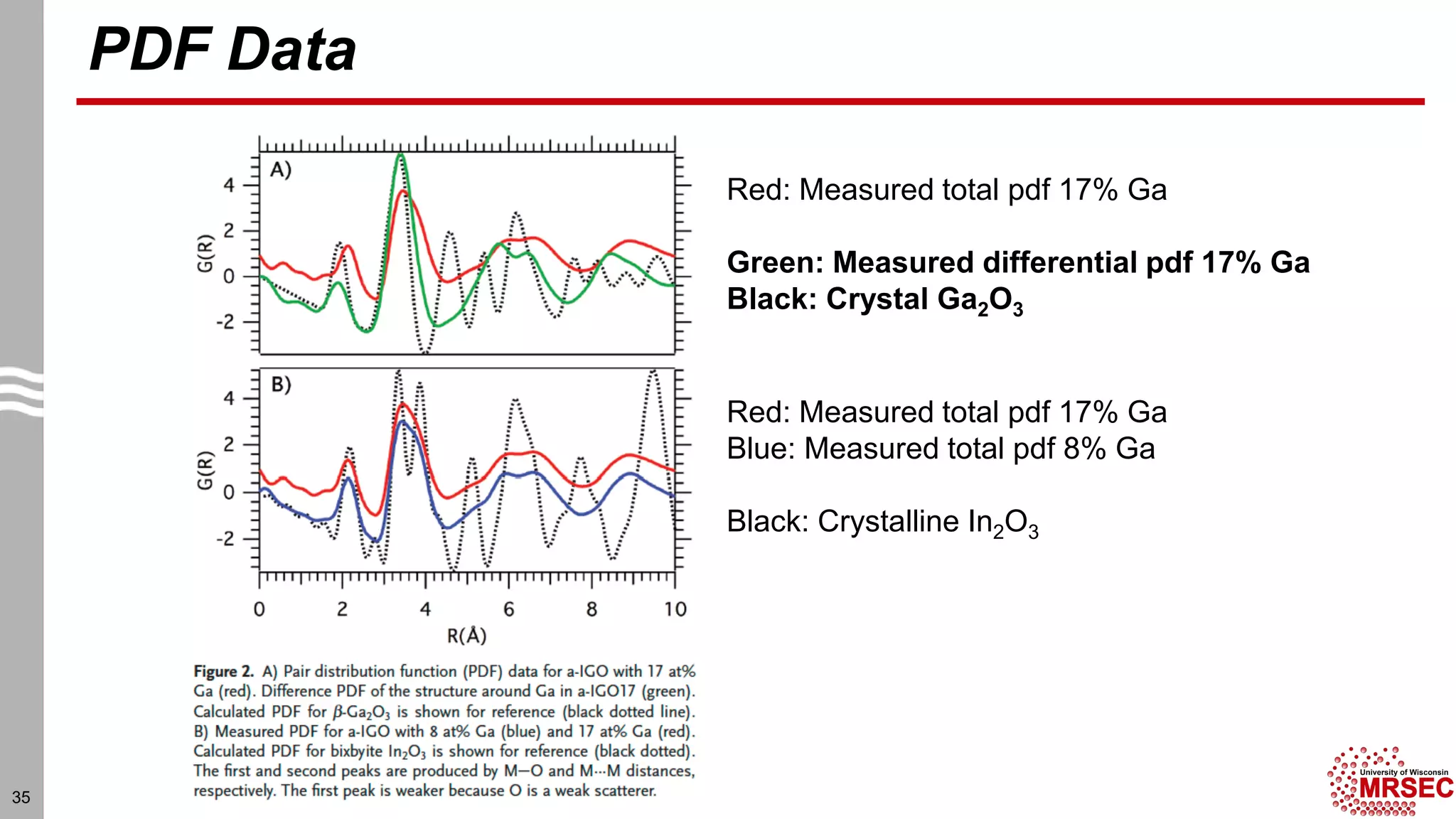
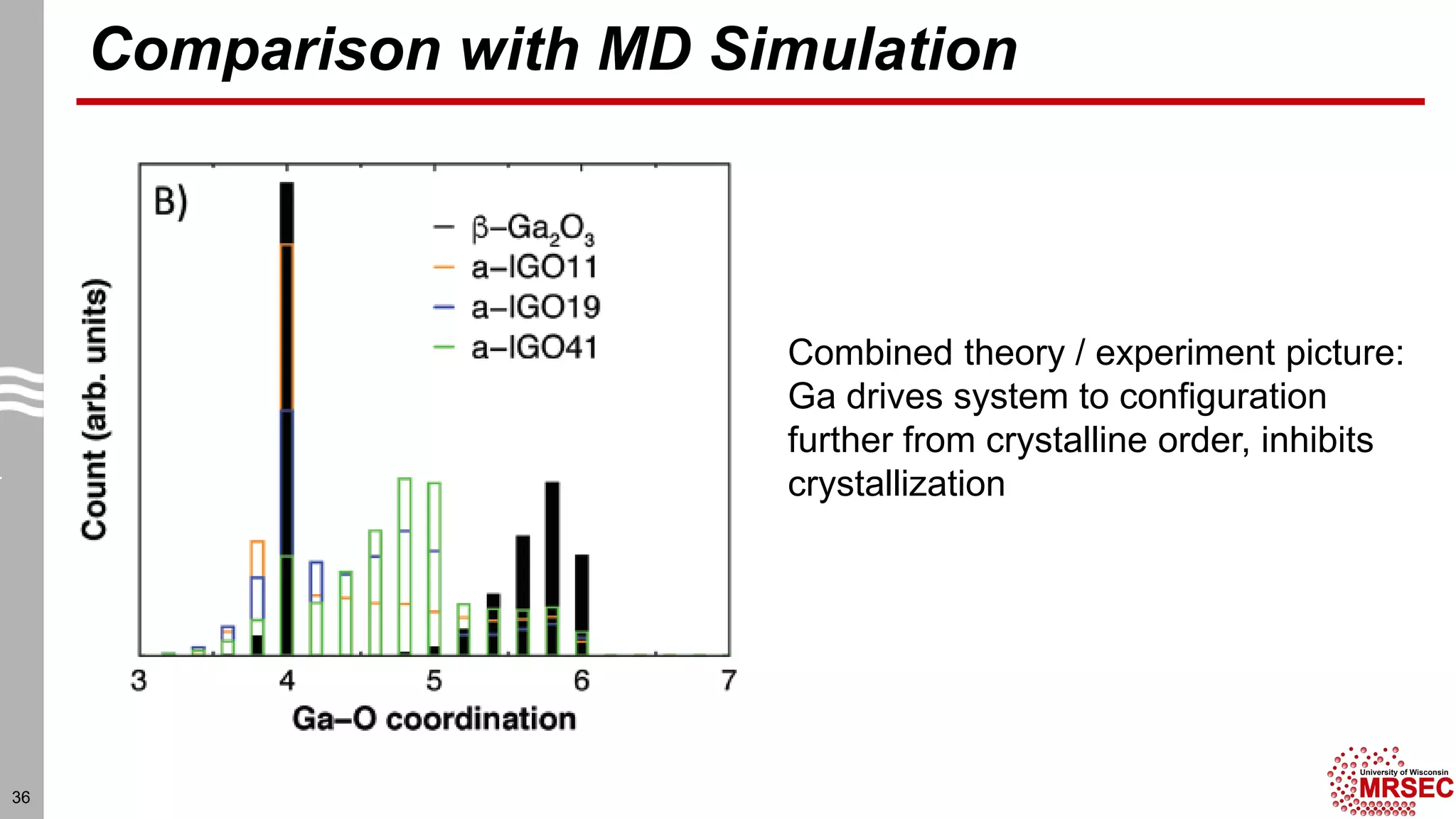
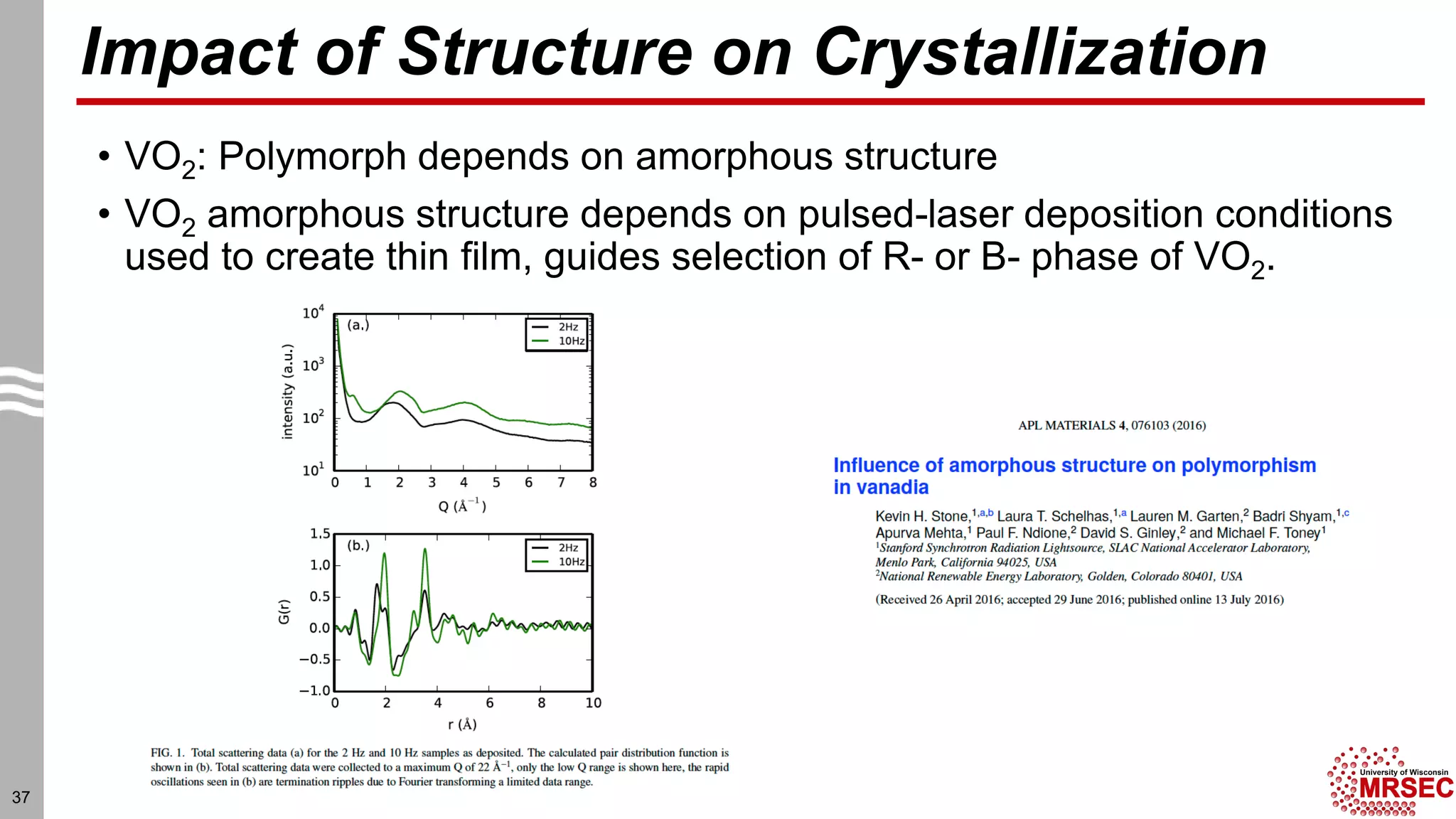
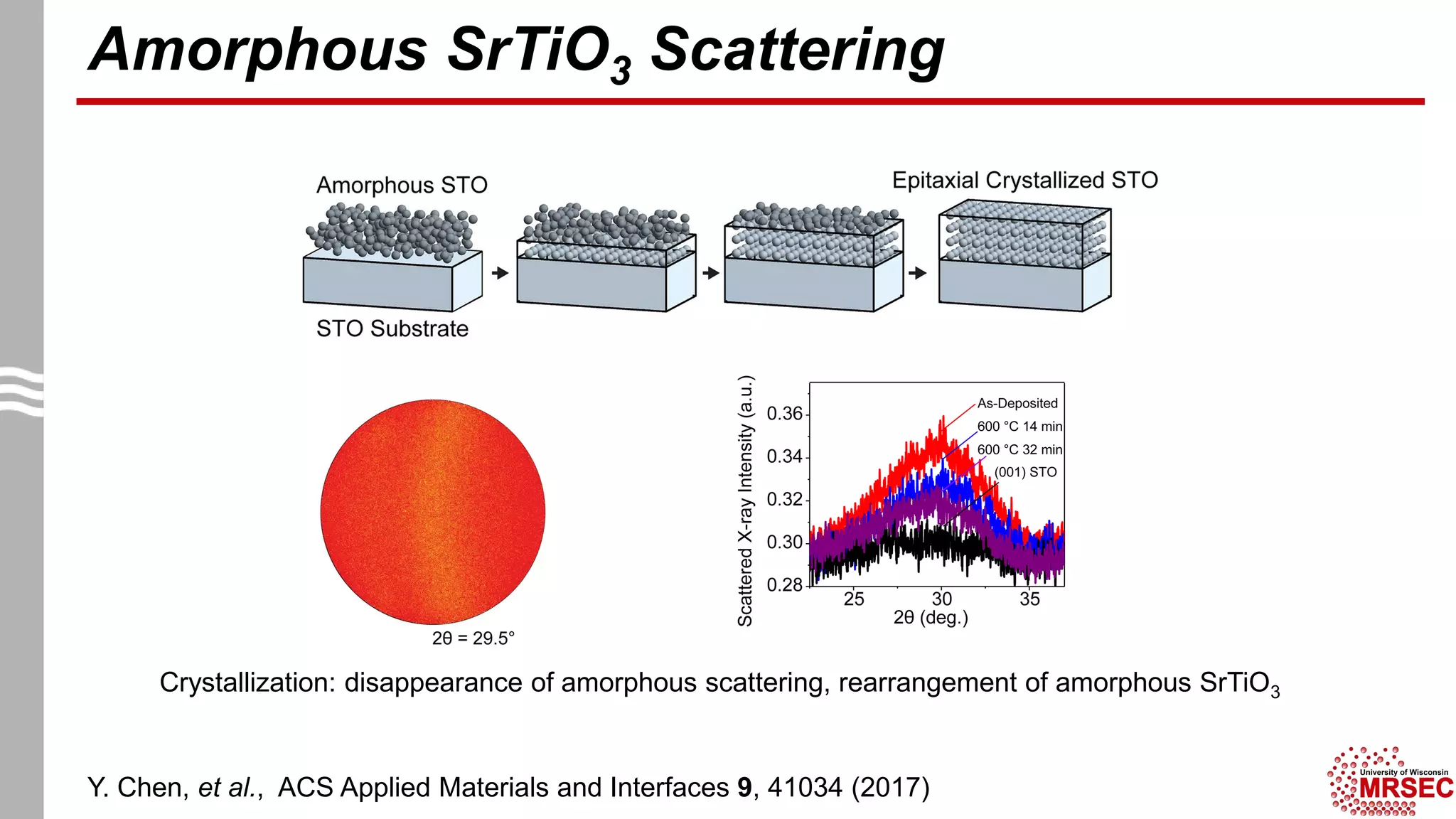
This document summarizes structural characterization techniques for amorphous materials. It discusses how amorphous materials have short-range order dominated by atomic bonding but no long-range translational order. Common characterization methods measure pair distribution functions to determine local structure. Structure varies between classes of amorphous materials like metallic glasses, molecular glasses, and covalent network glasses.