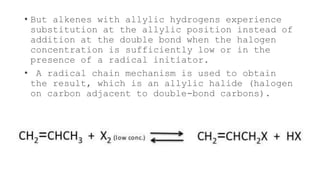
The allylic halogenation reaction involves the substitution of a halogen atom at the allylic position of an alkene. This occurs through a radical chain mechanism initiated by a small amount of halogen radical produced from NBS or heat. The halogen radical abstracts an allylic hydrogen to form a resonance-stabilized radical intermediate. This intermediate then reacts with a halogen molecule to form the allylic halide product and regenerate the halogen radical. Allylic chlorination and bromination are important industrial reactions for producing compounds such as 3-chloropropene.