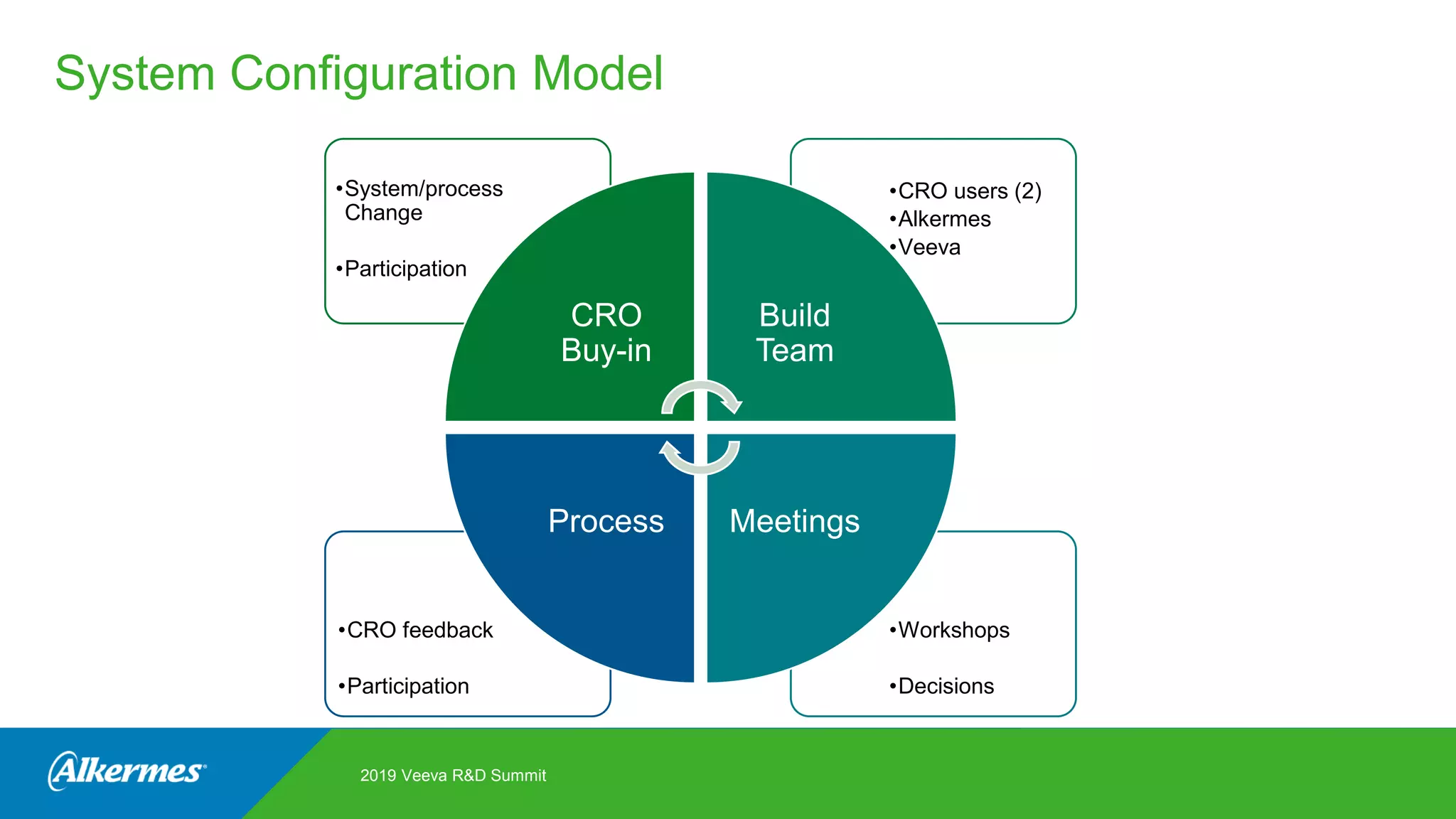
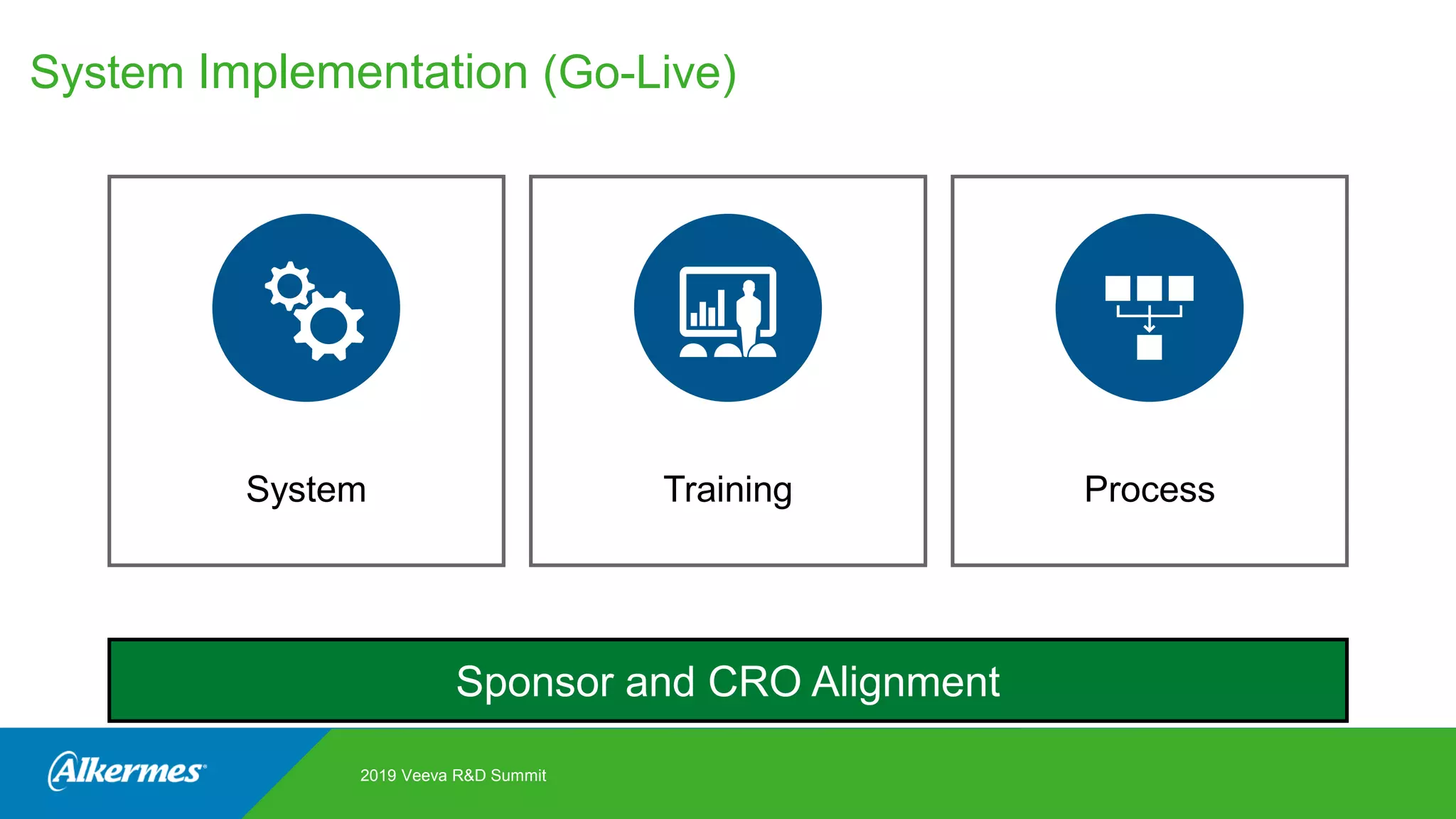
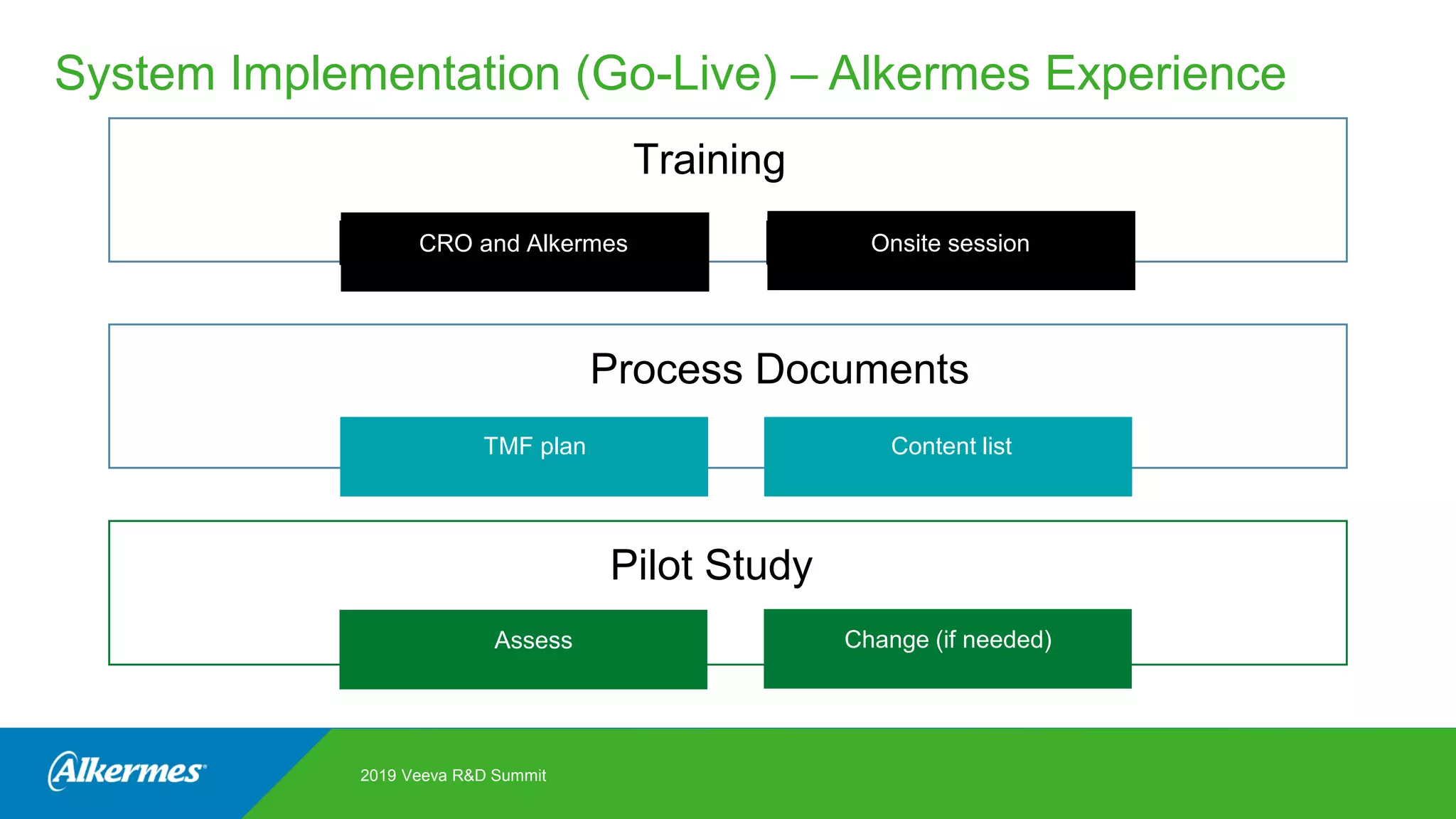
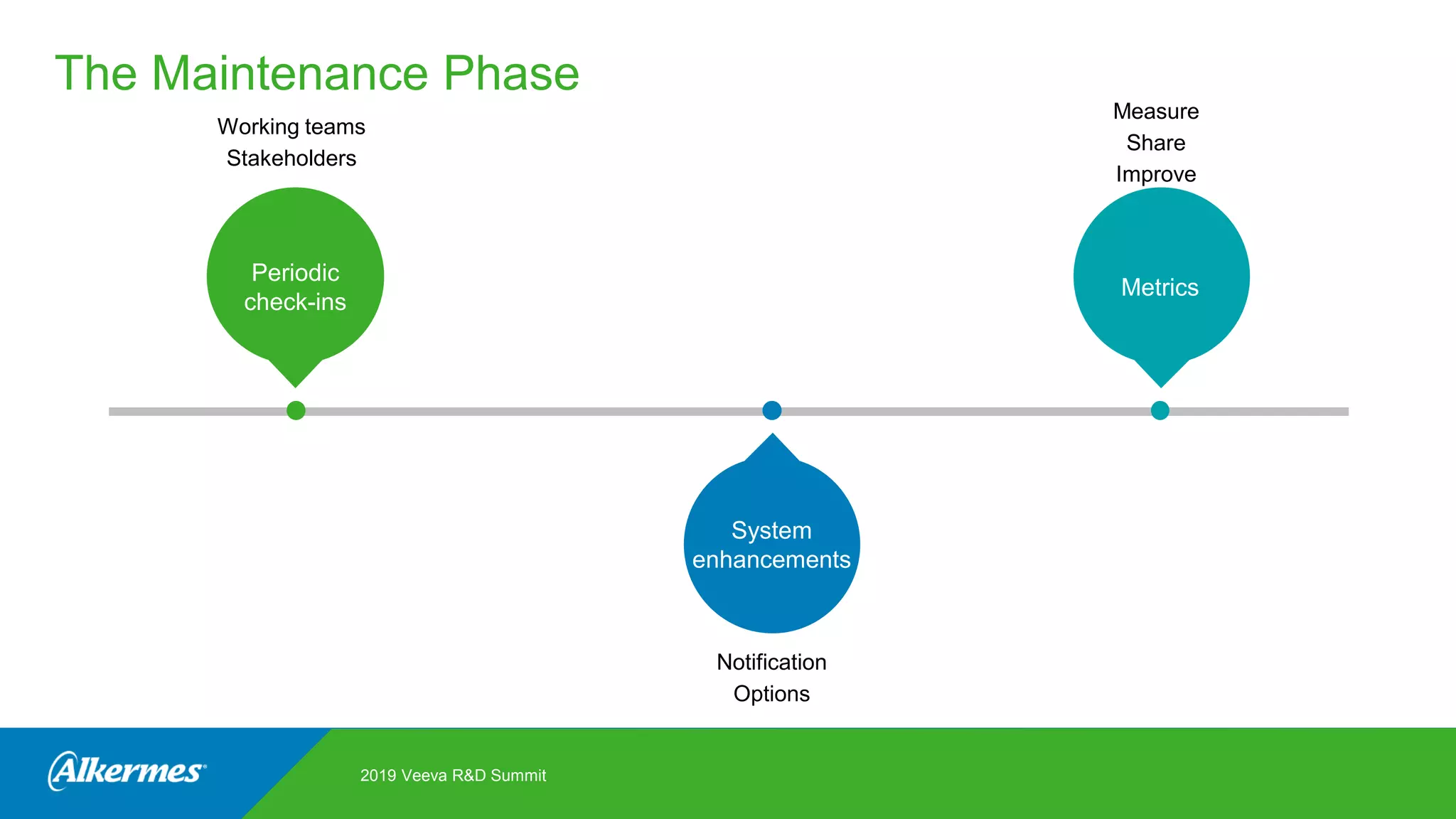
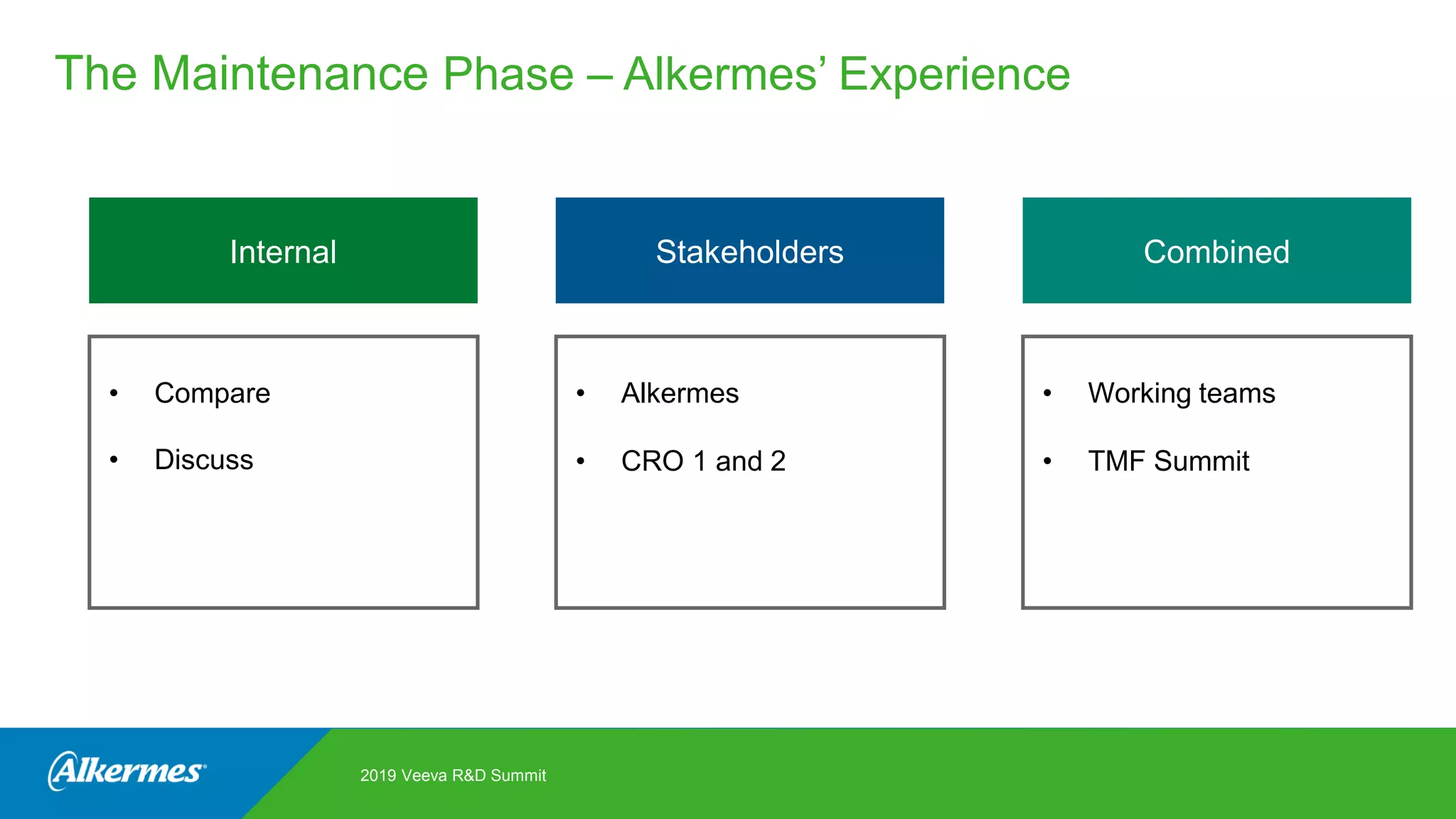
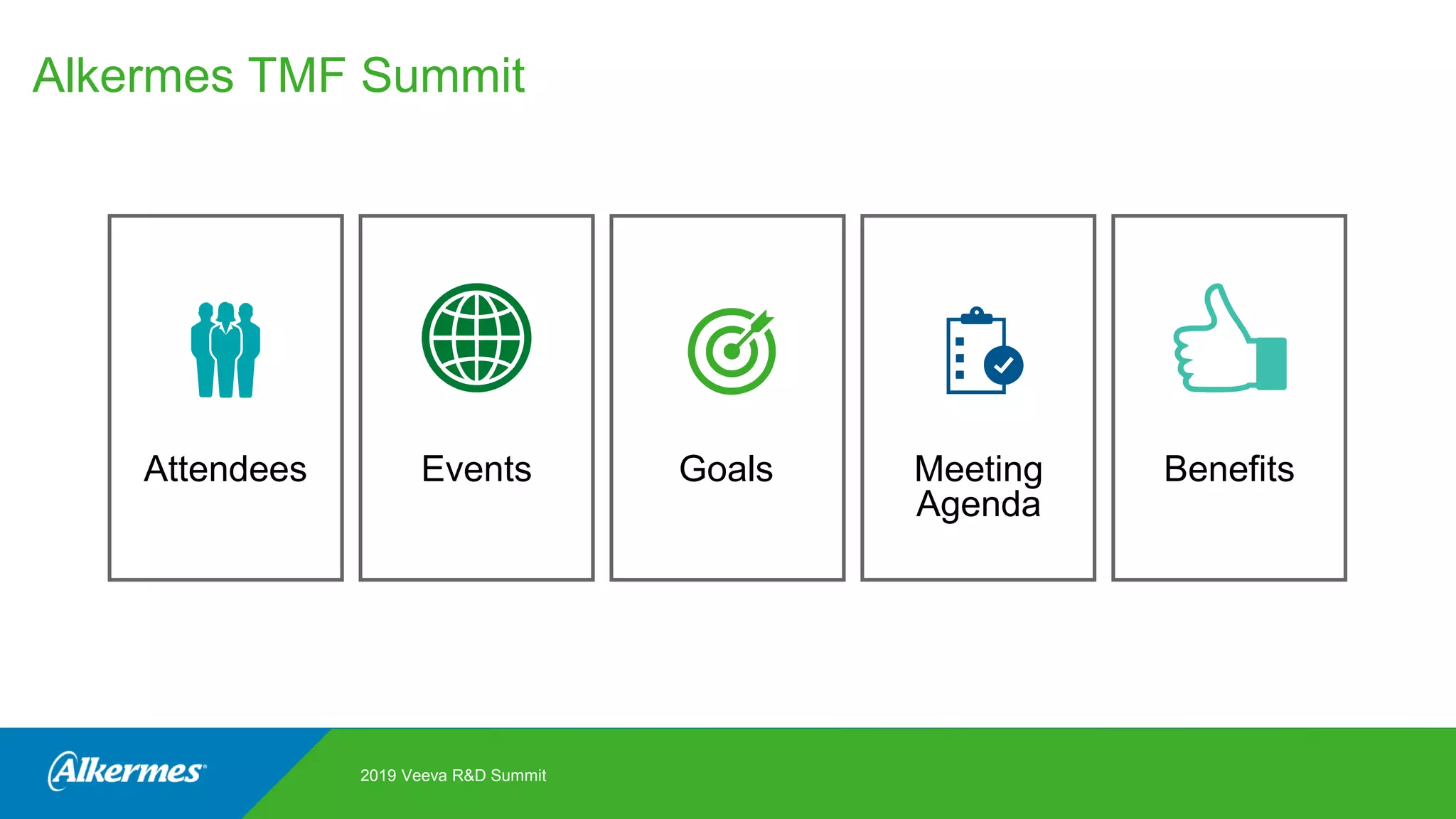
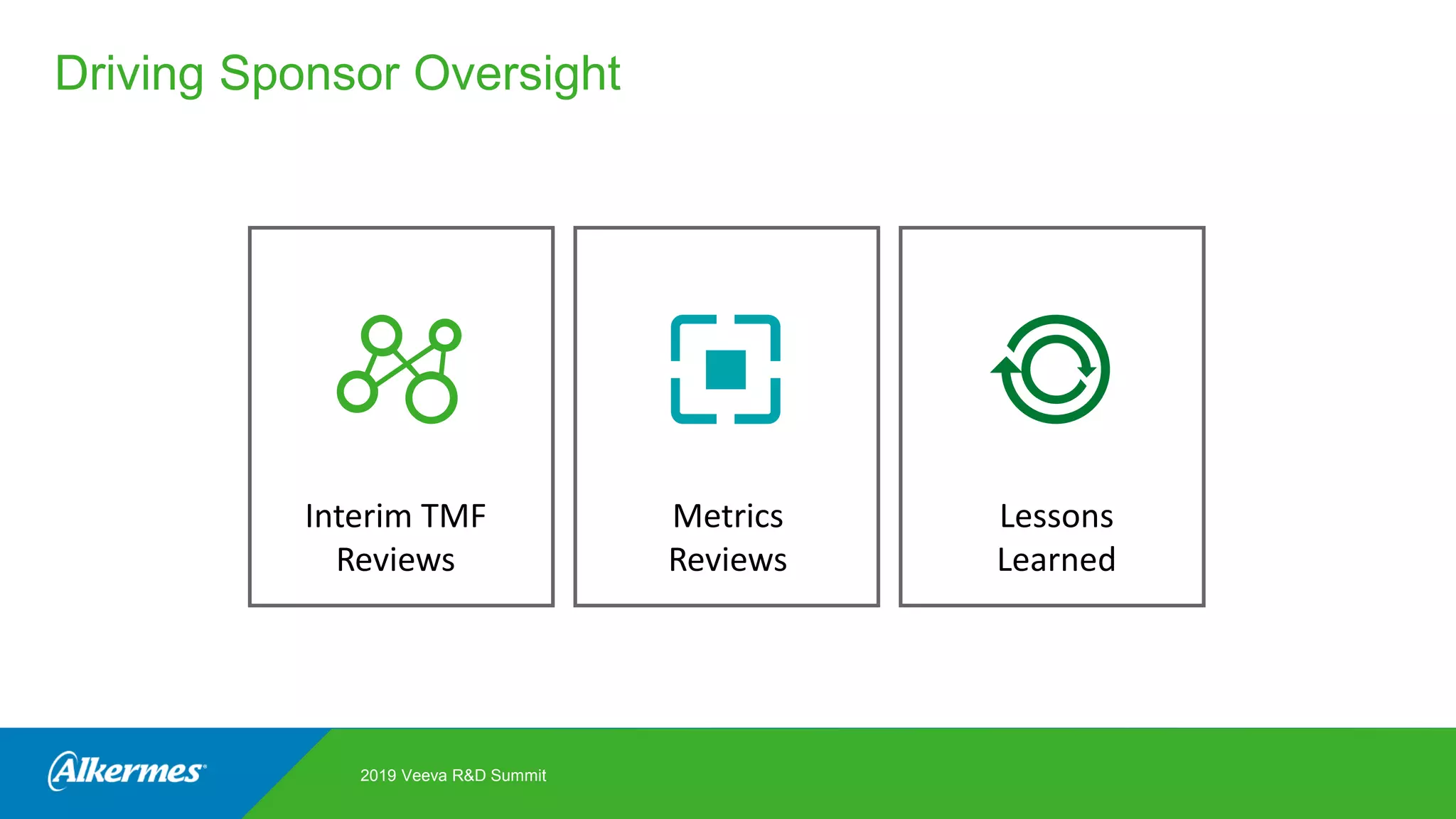
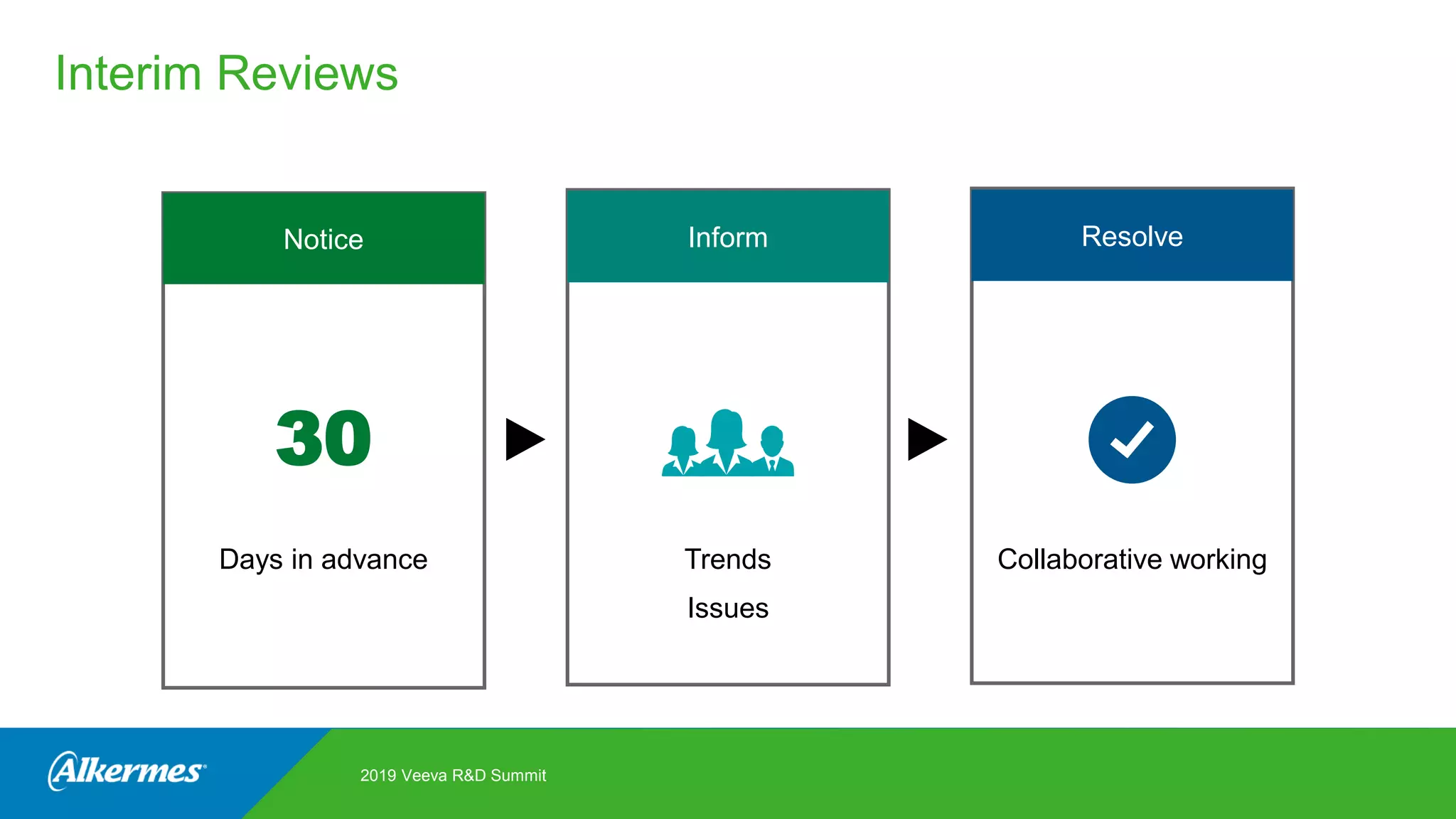
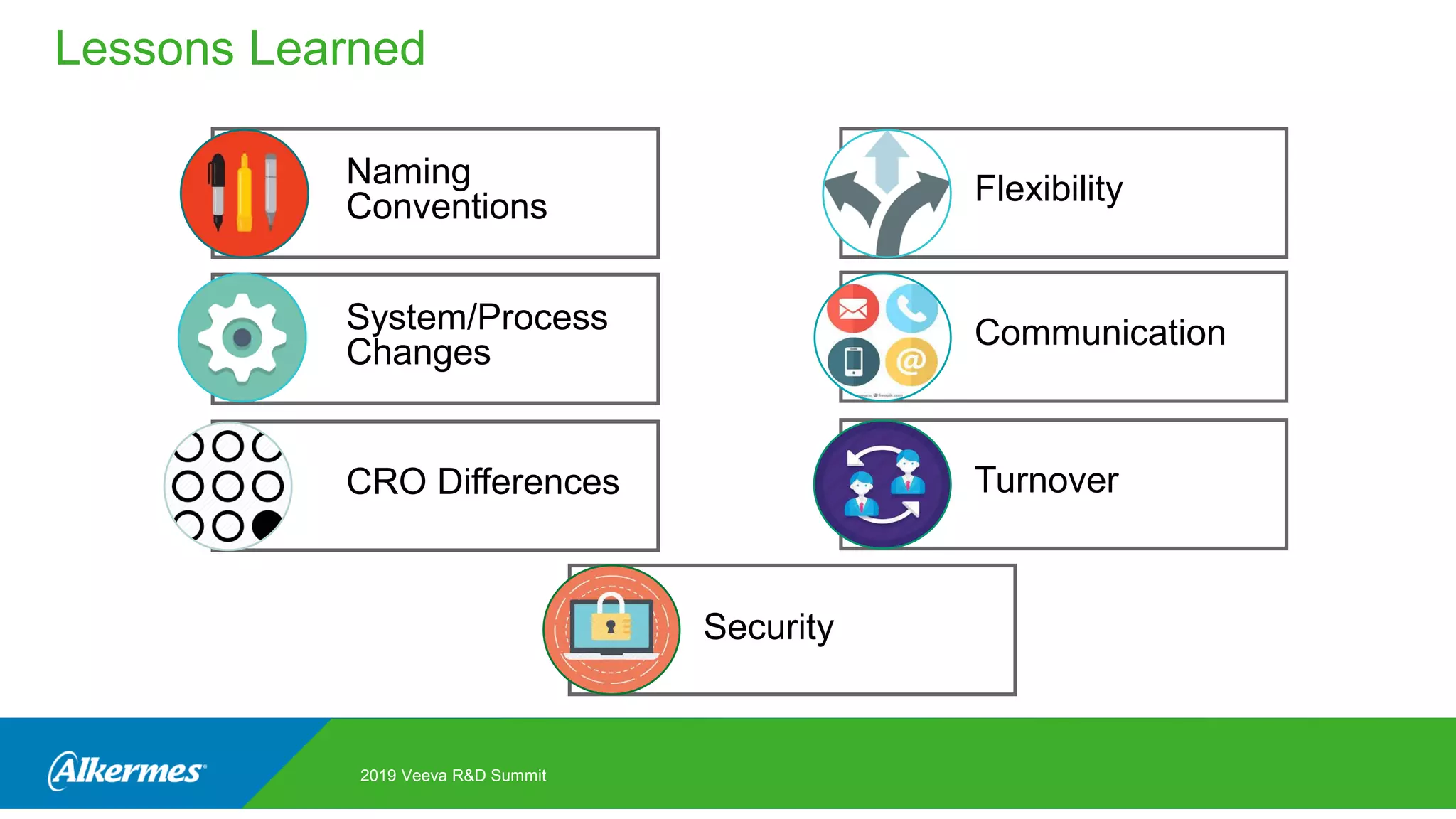
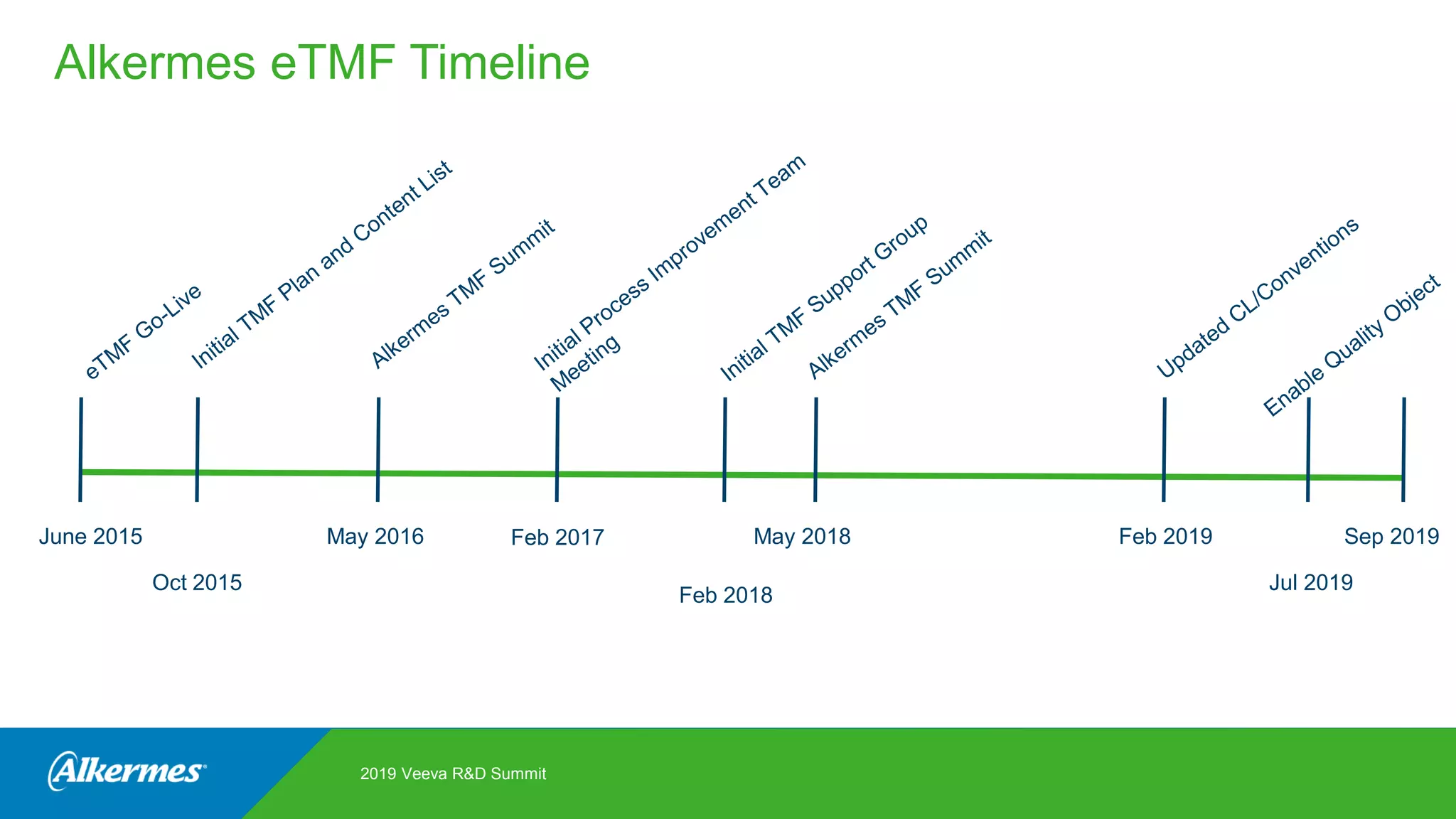
Kate Santoro from Alkermes presented on their experience improving TMF quality and oversight through effective collaboration with CROs on their Veeva eTMF system. Key aspects of their process included getting CRO input during system configuration, training CRO and sponsor users together, aligning processes during implementation, and holding periodic check-ins and TMF summits with internal and CRO stakeholders to review metrics and identify areas for improvement. The goal was to drive better sponsor oversight through collaborative working relationships with CROs.