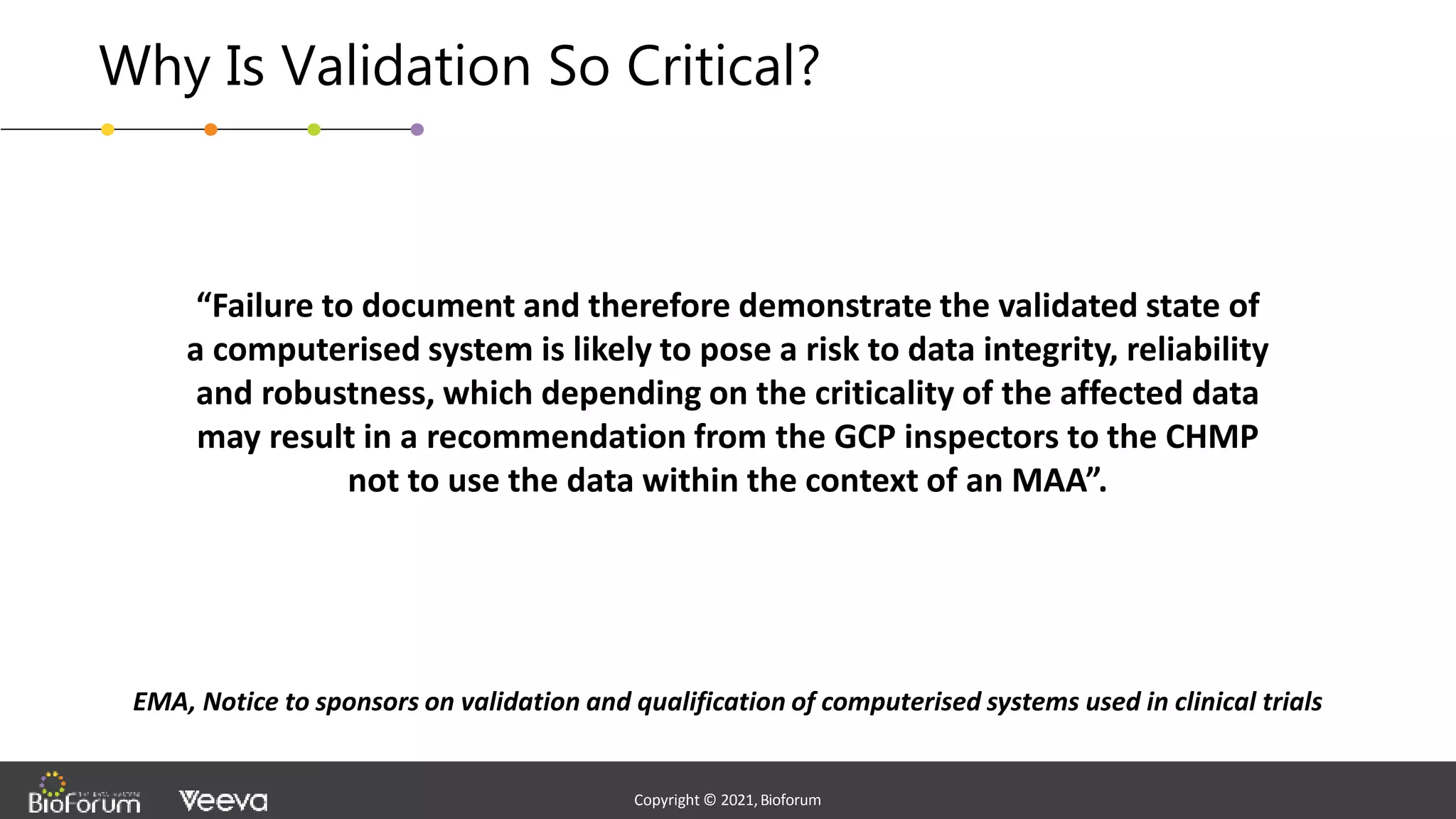
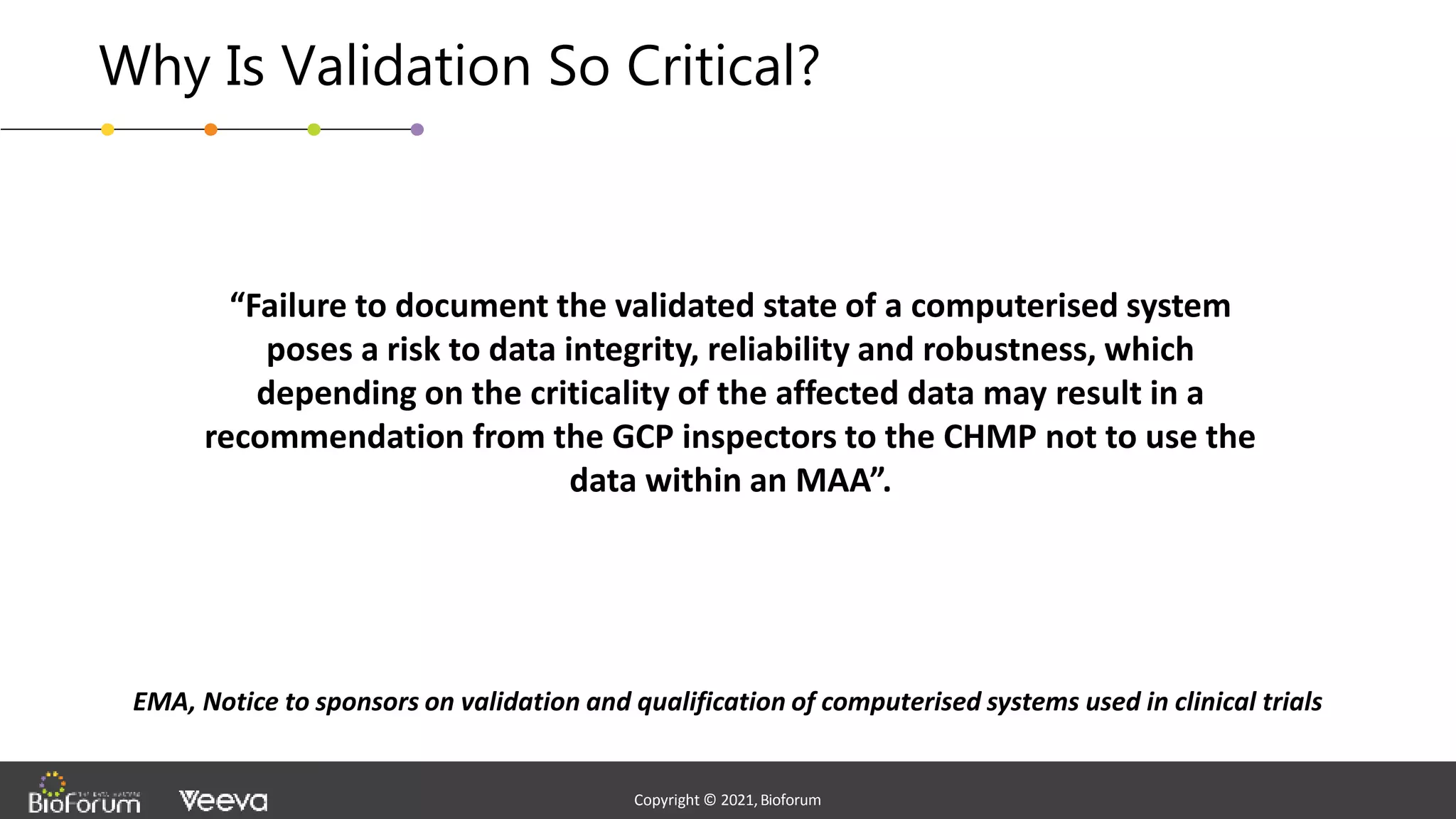
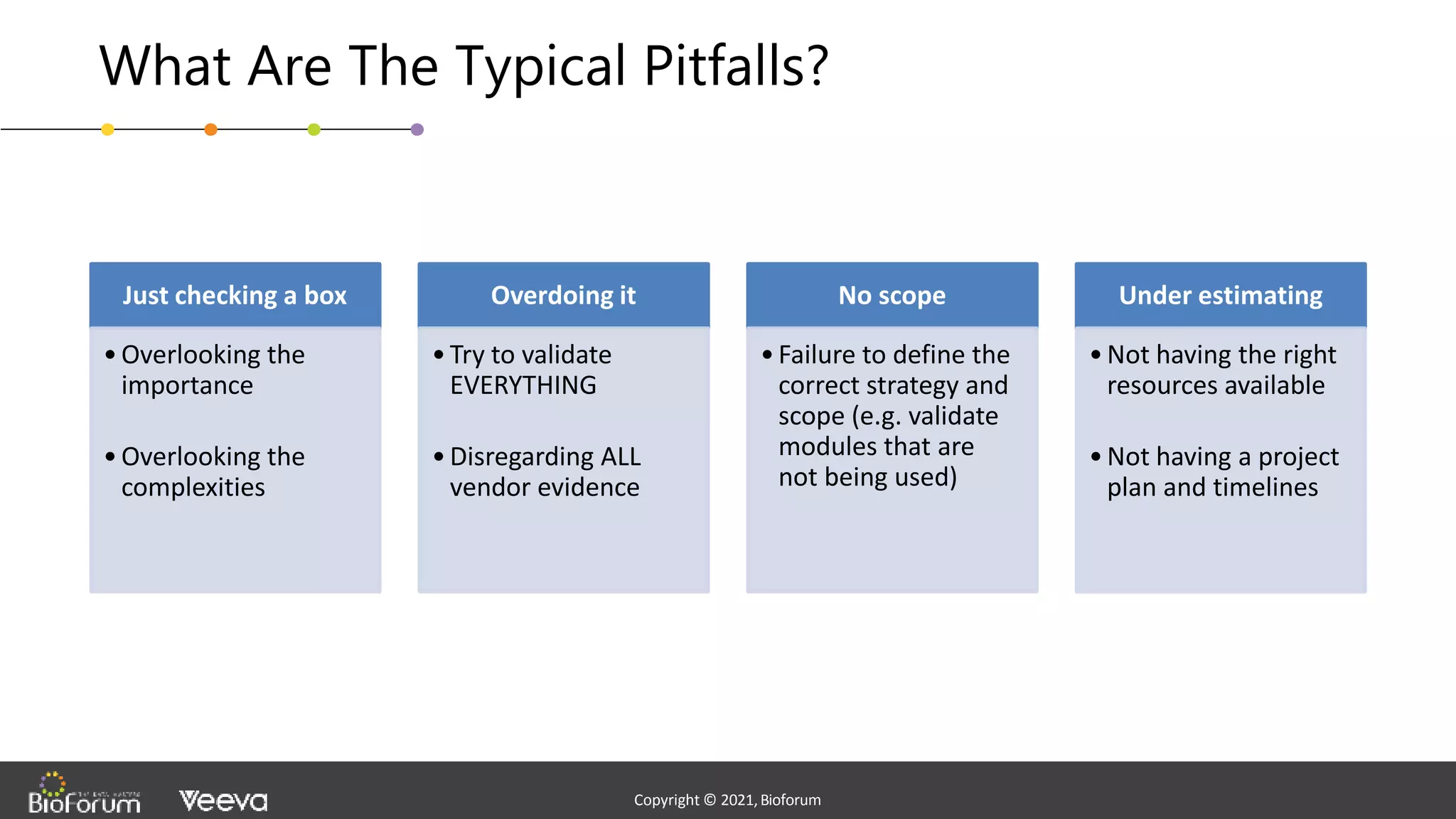
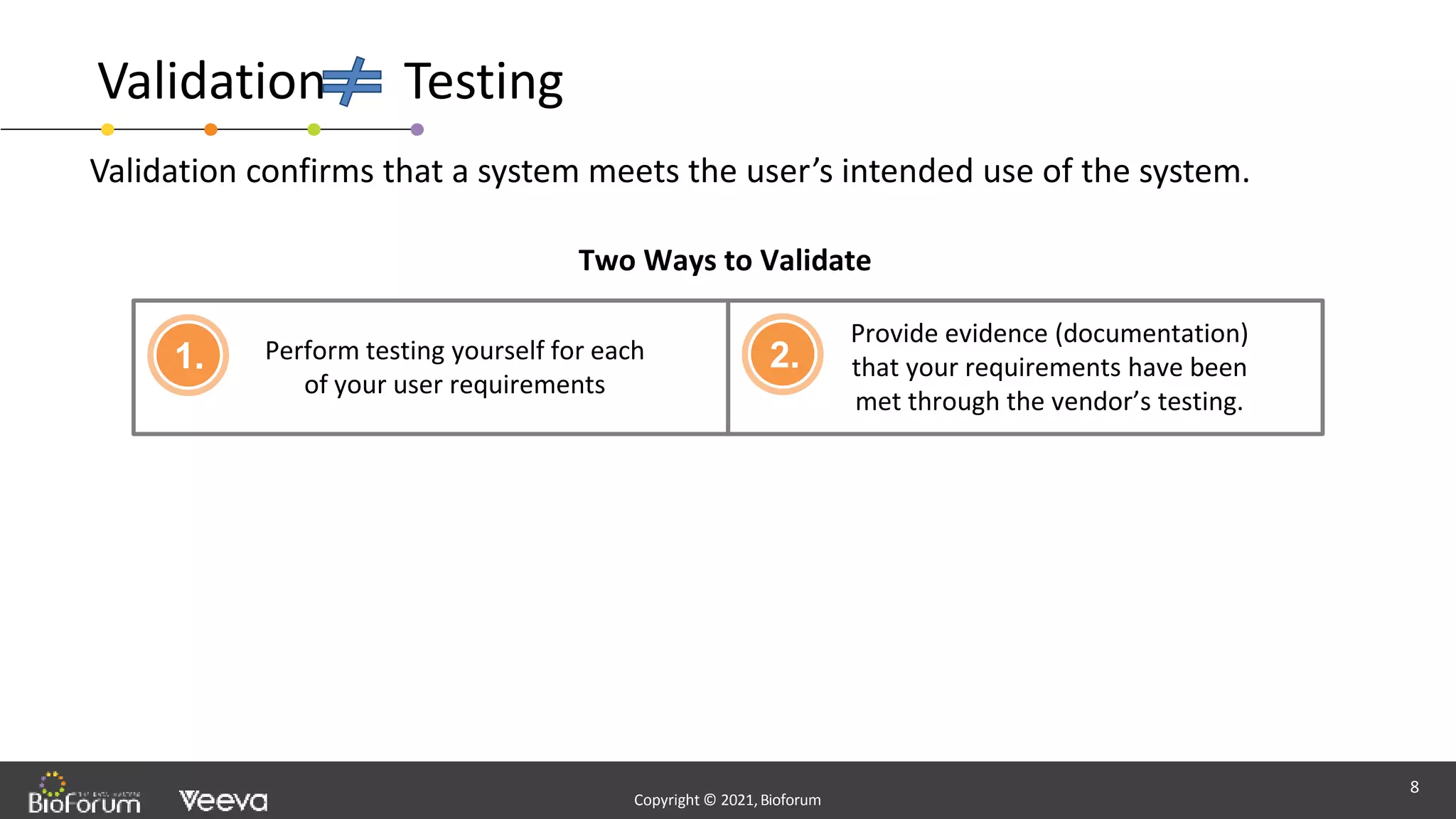
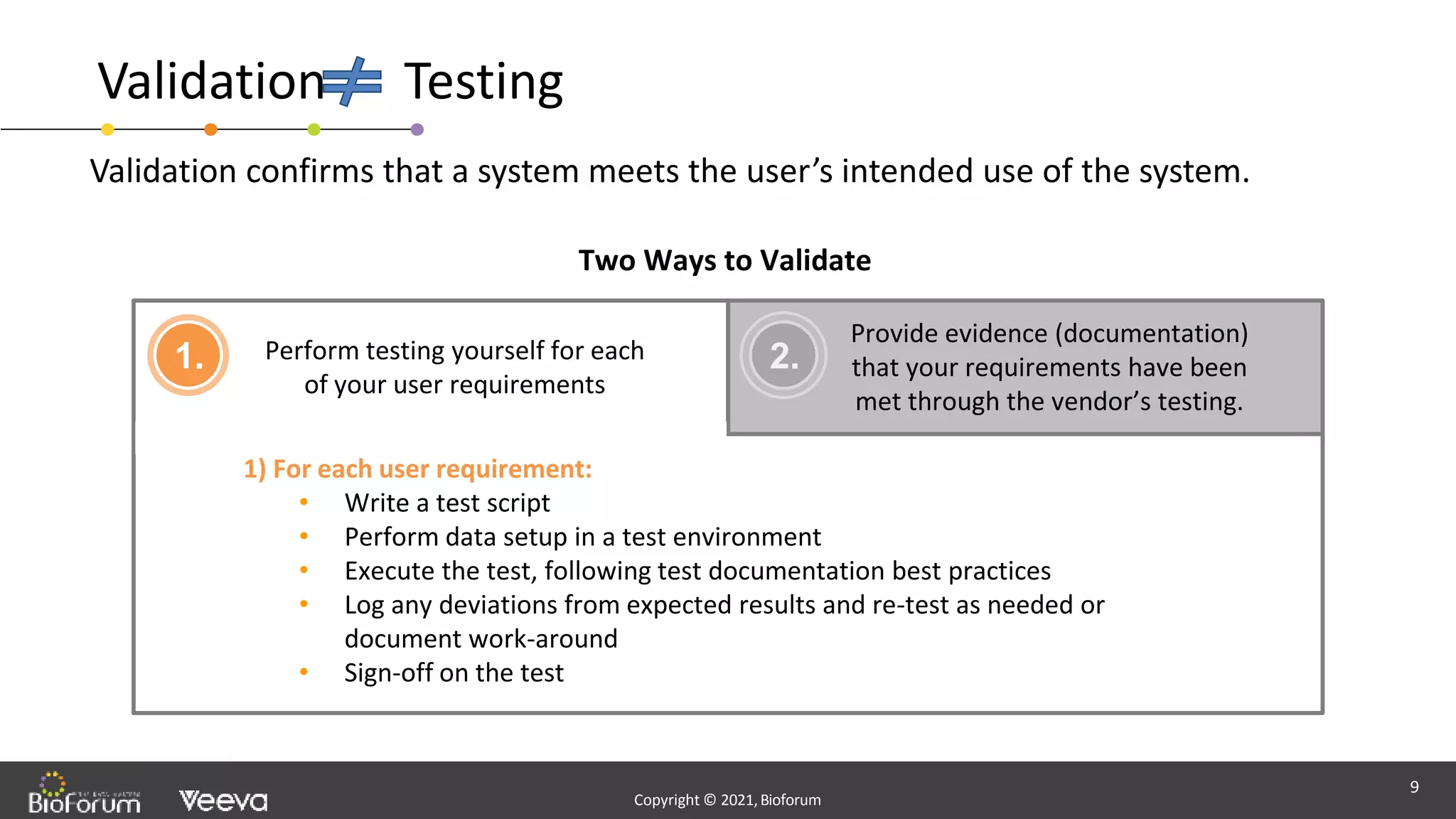
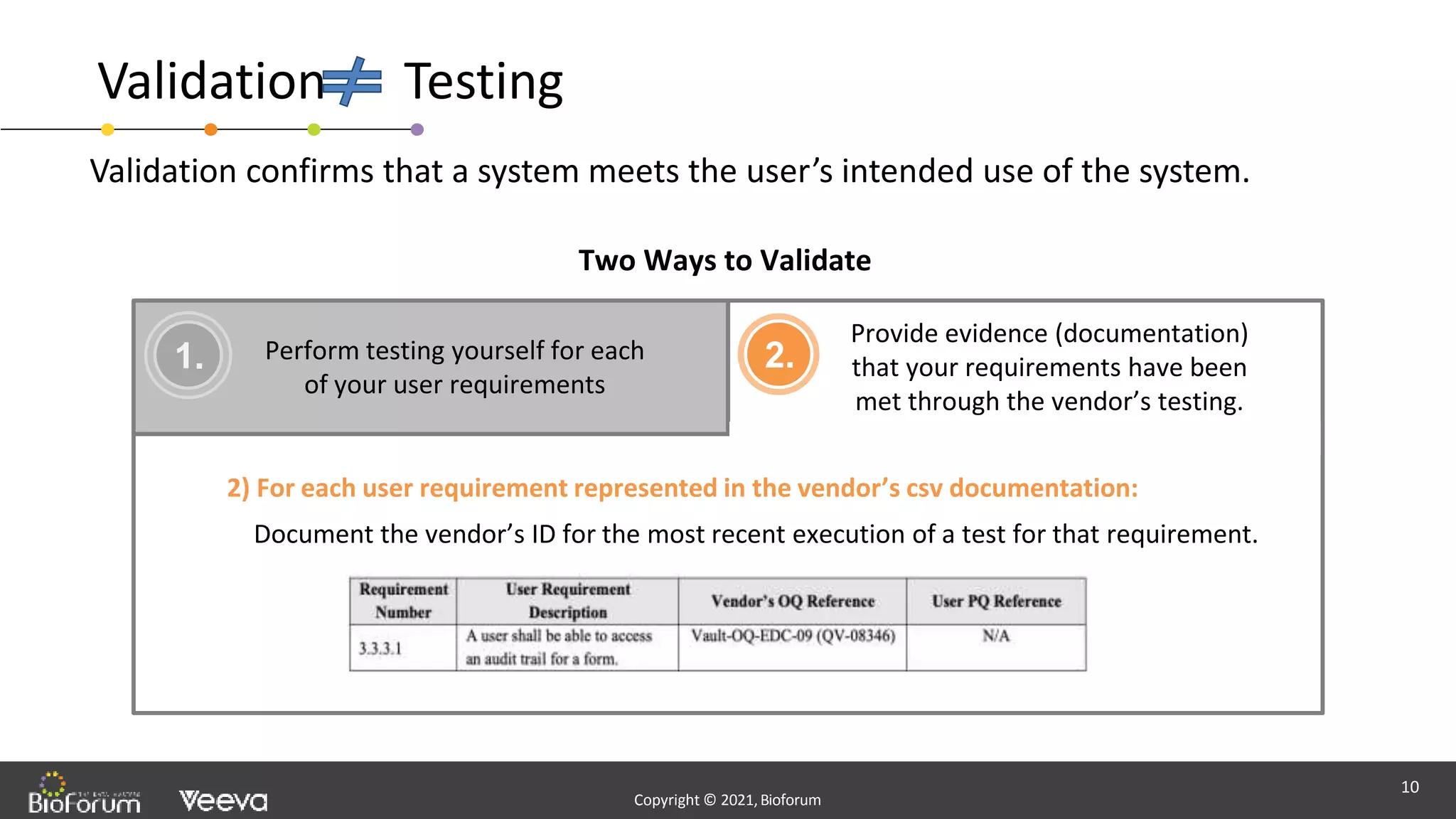
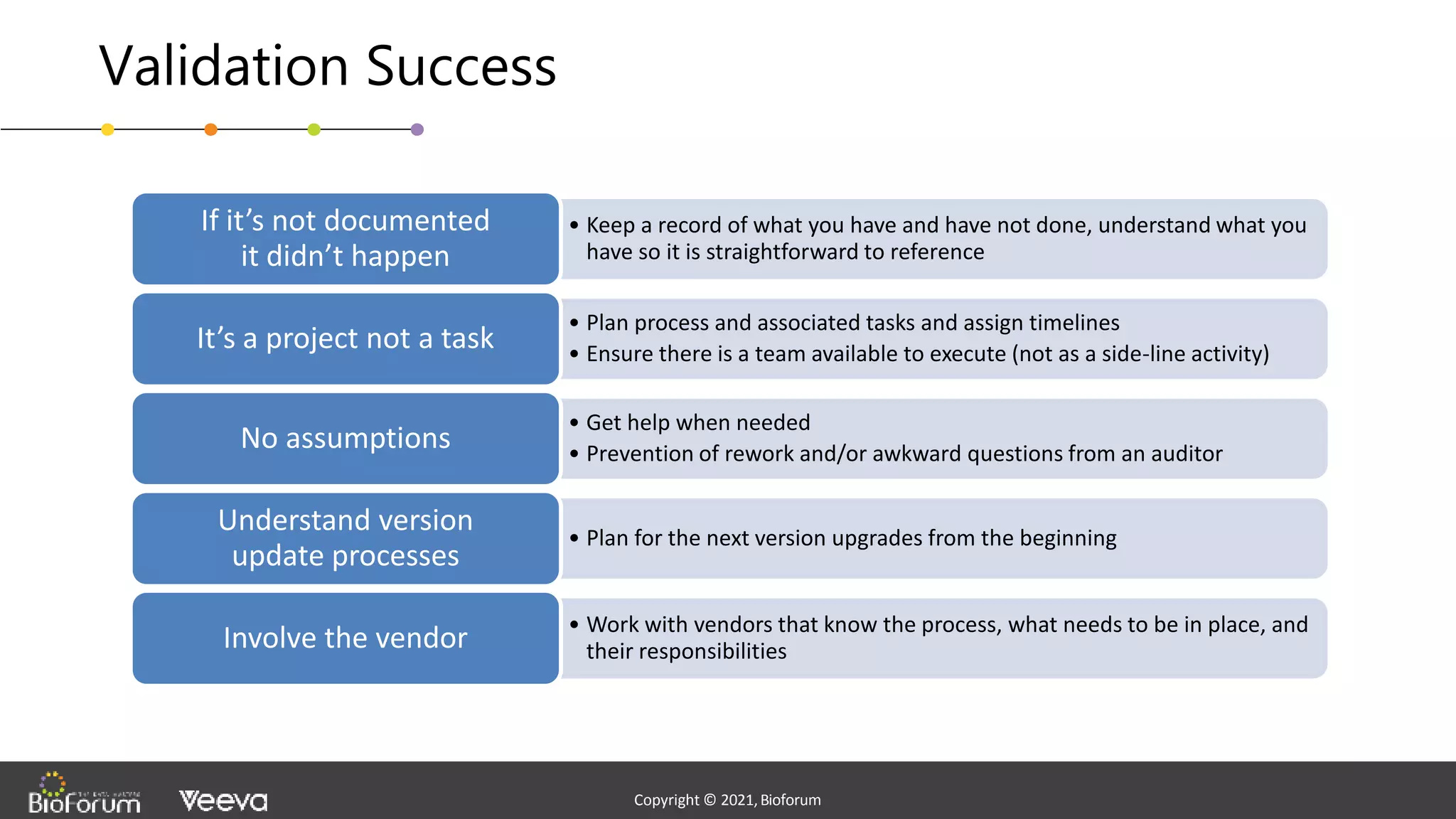
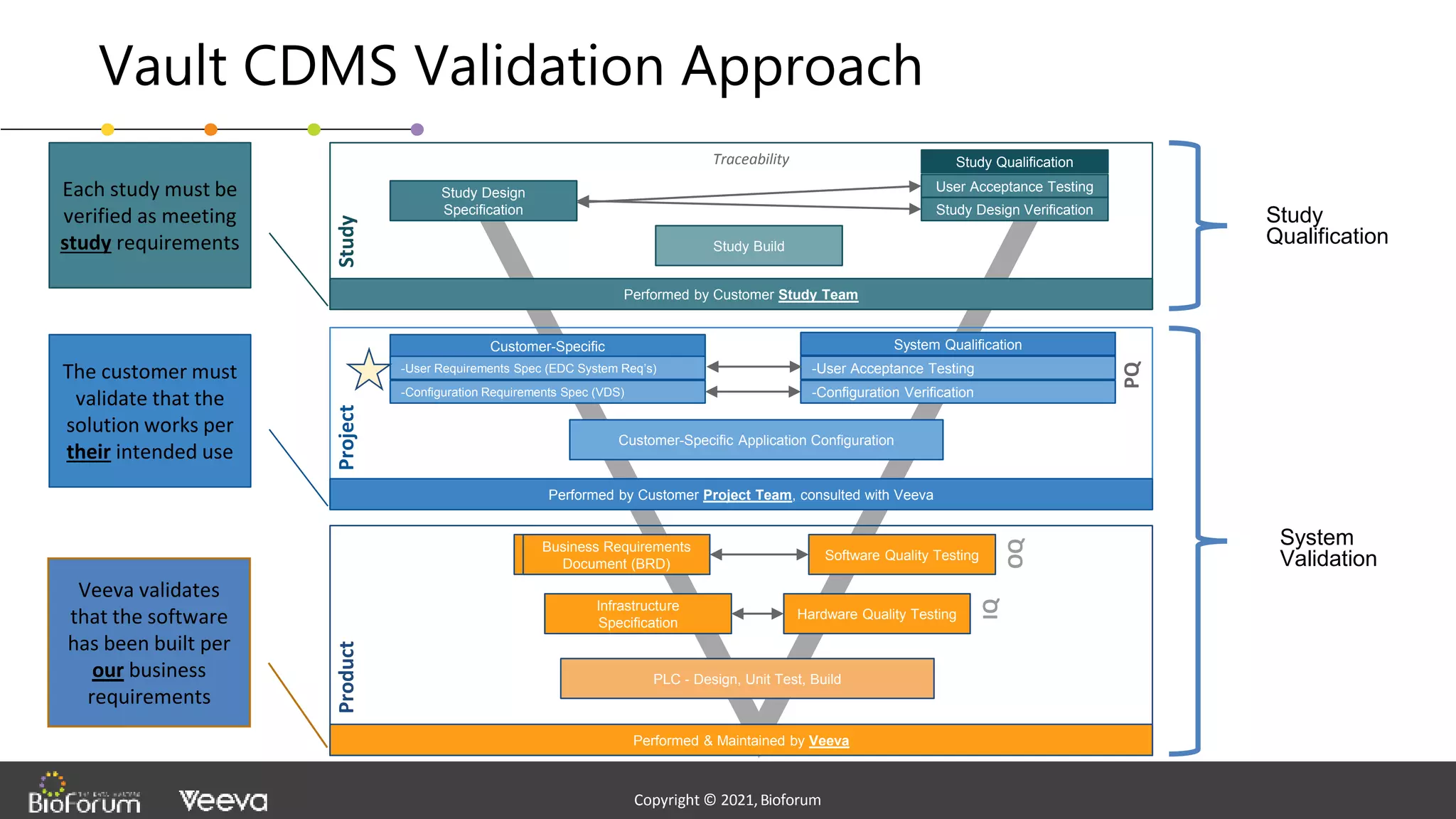
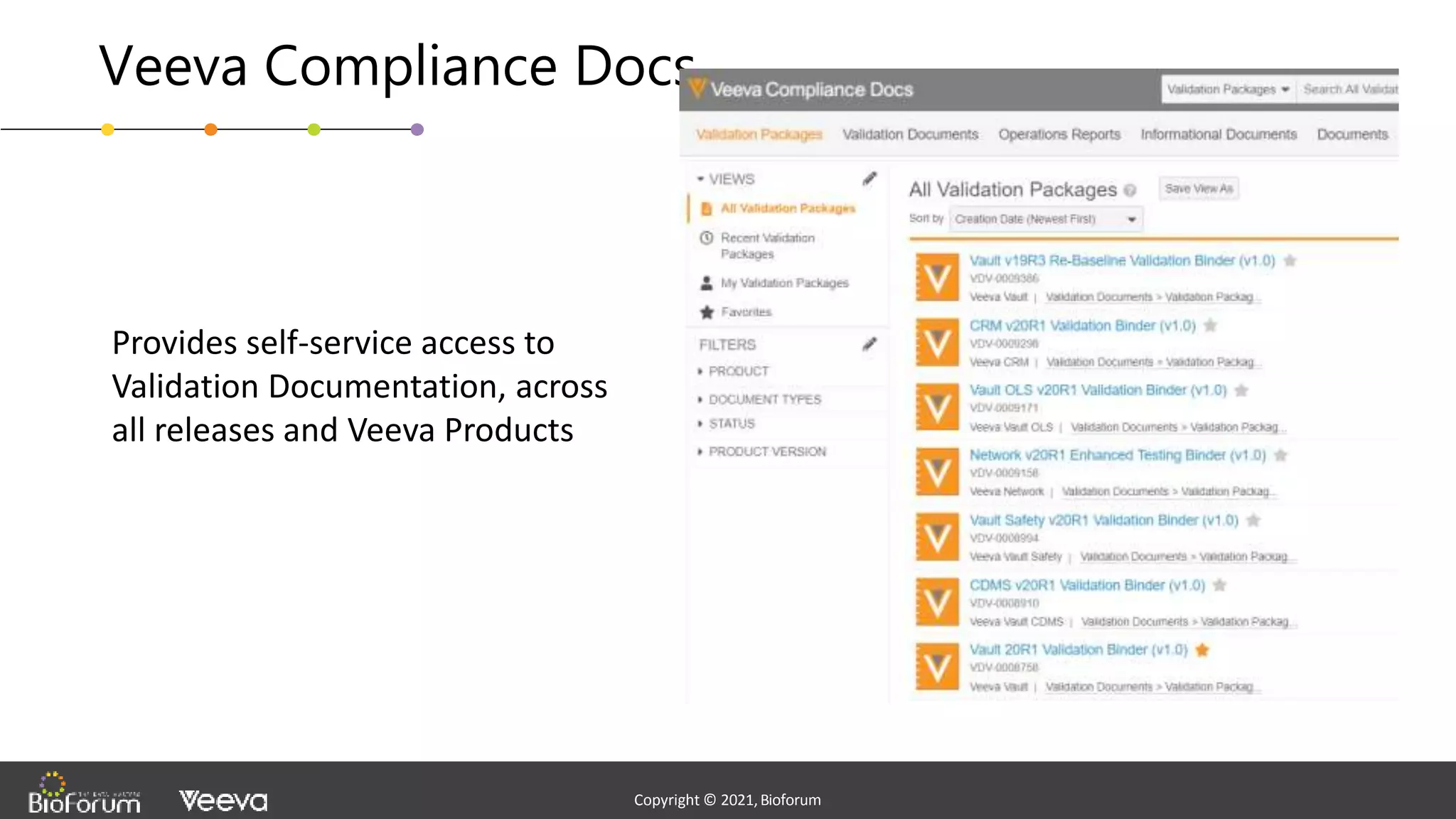
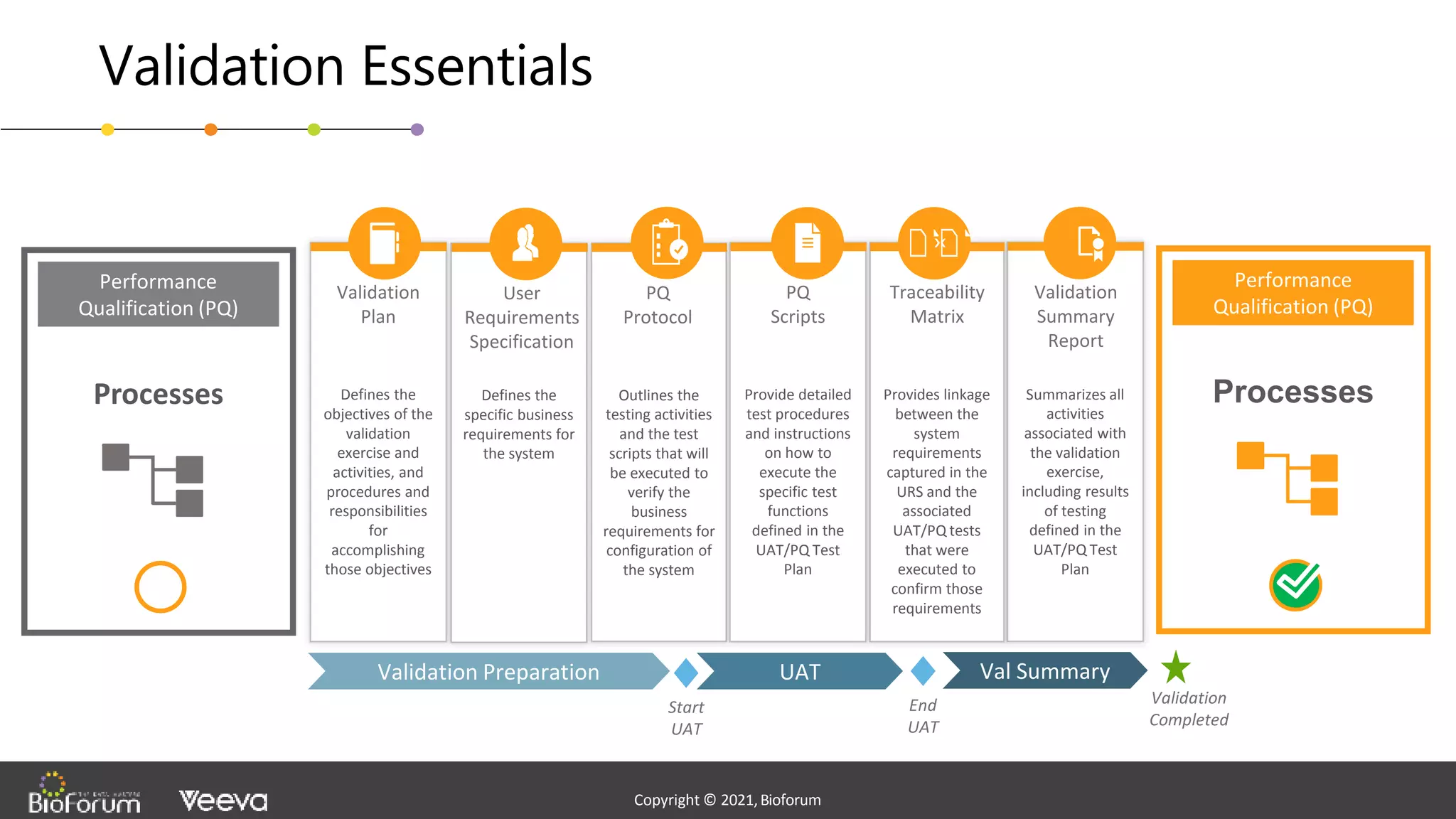
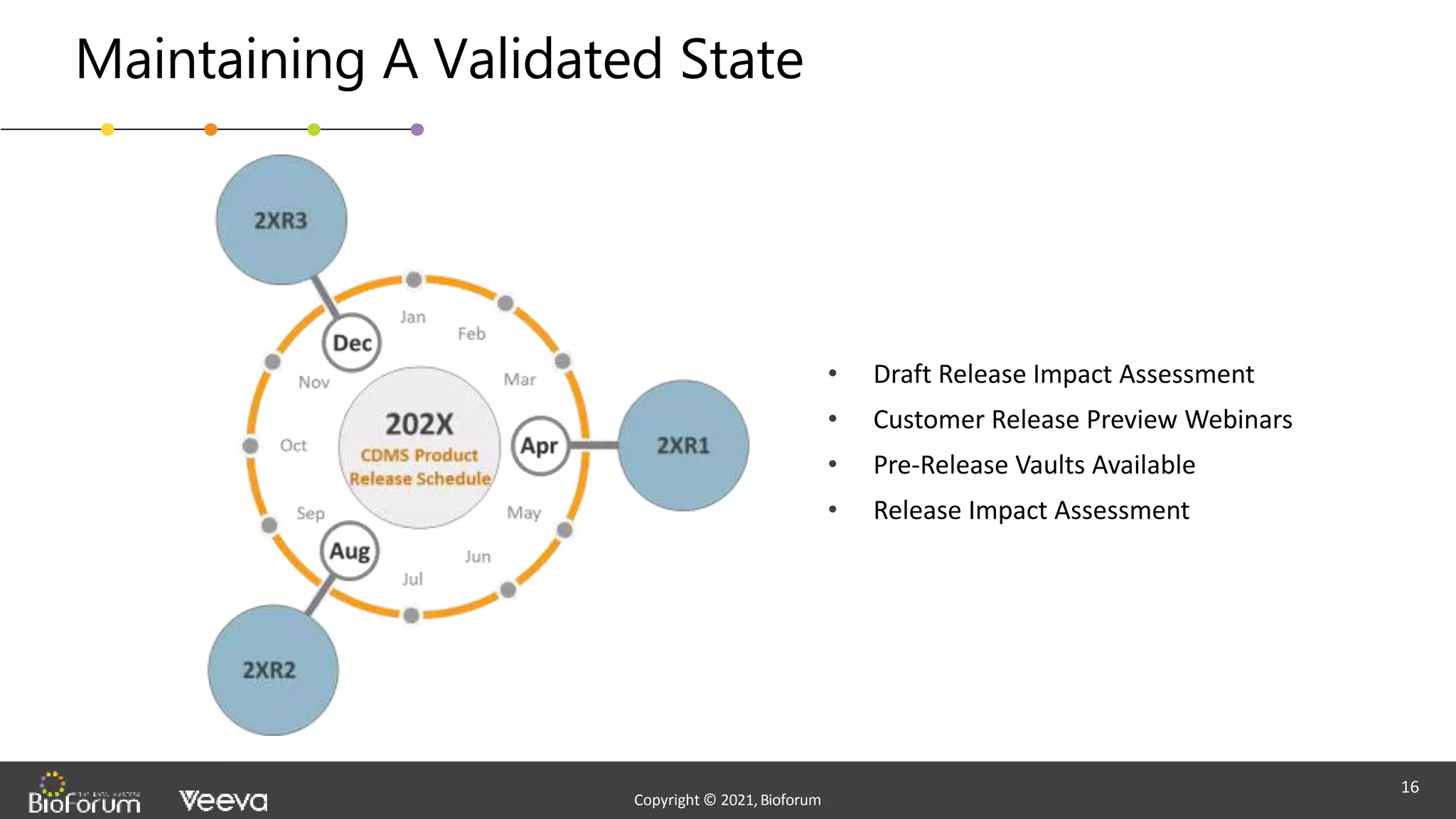
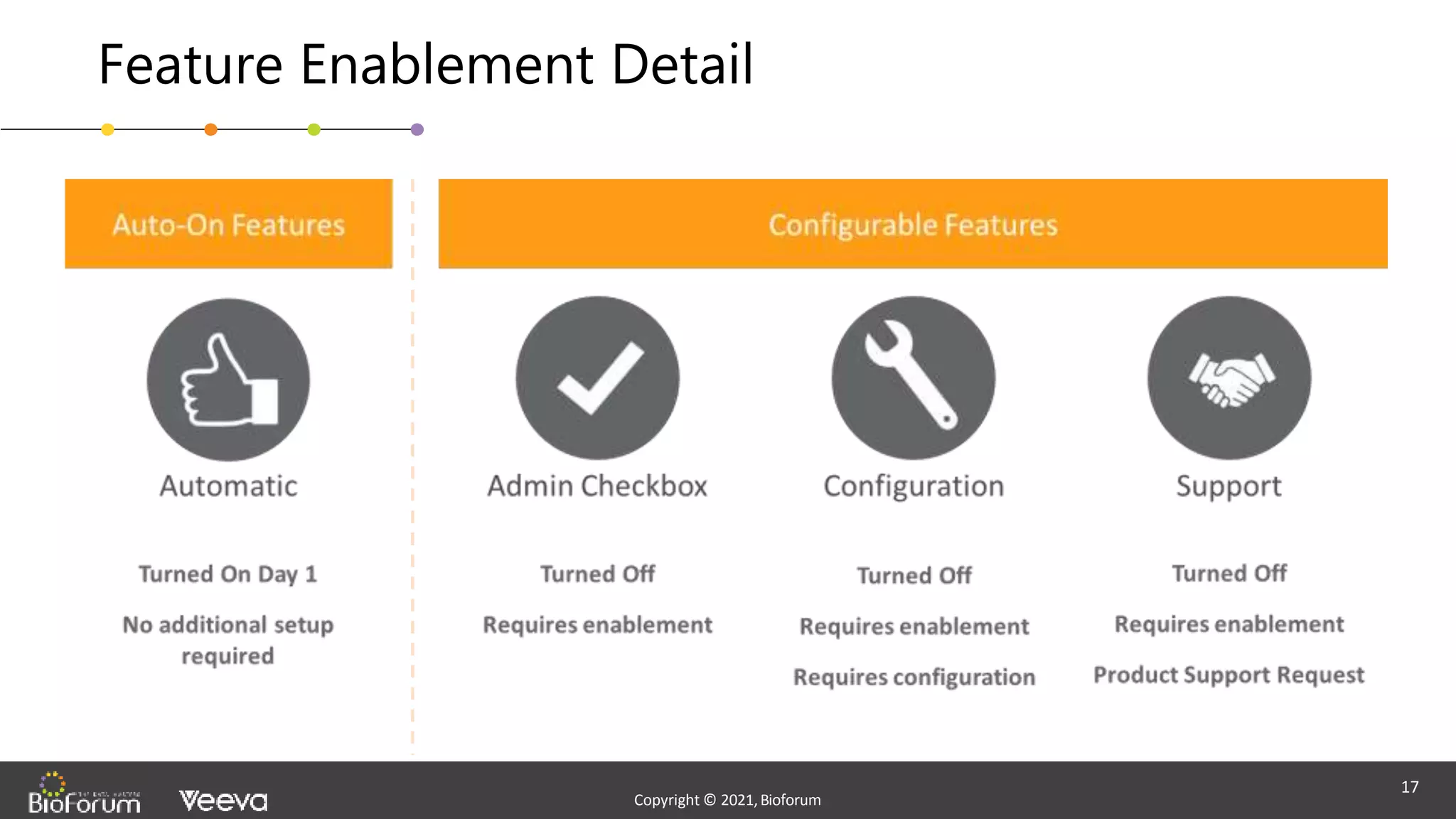
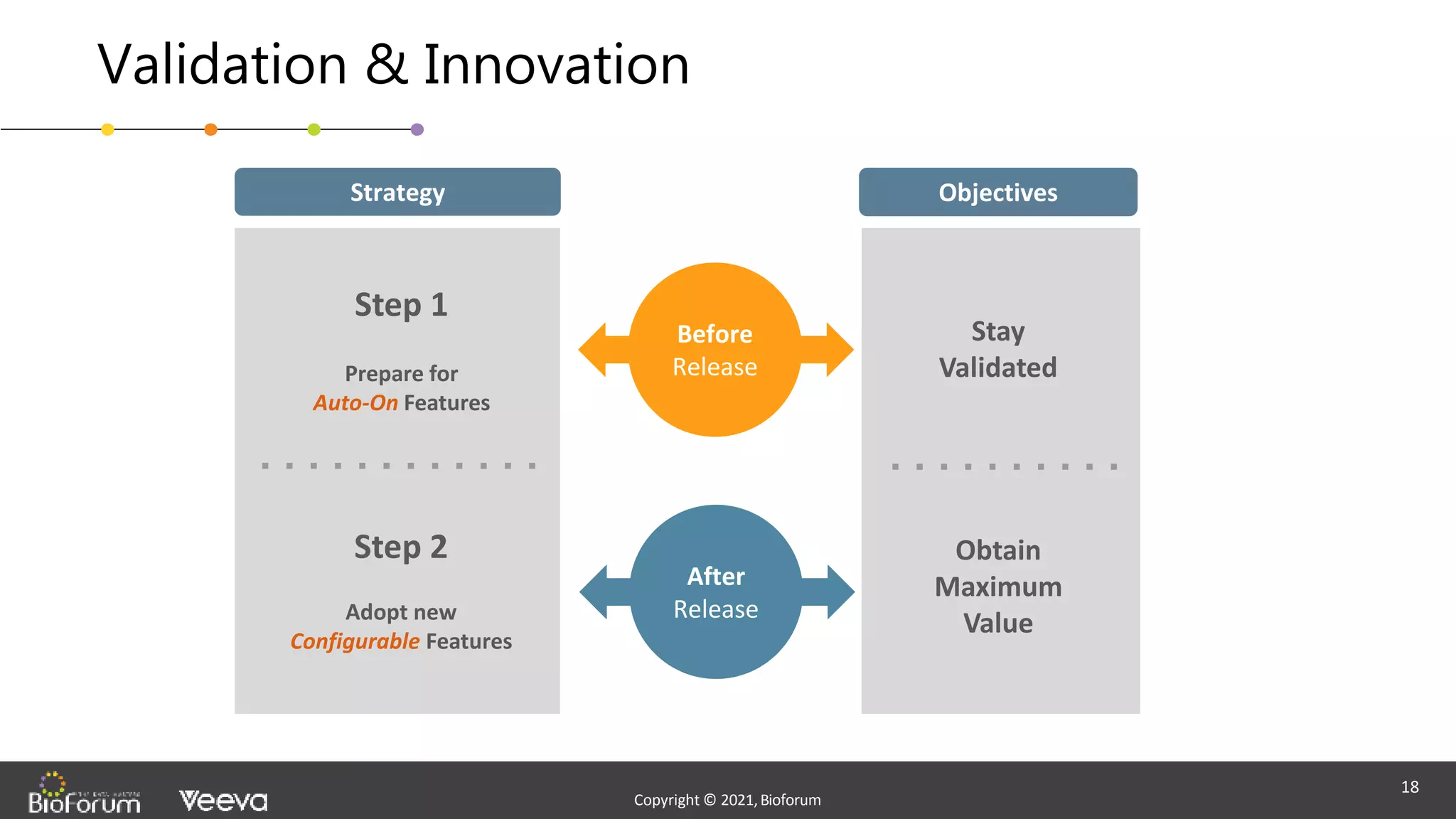
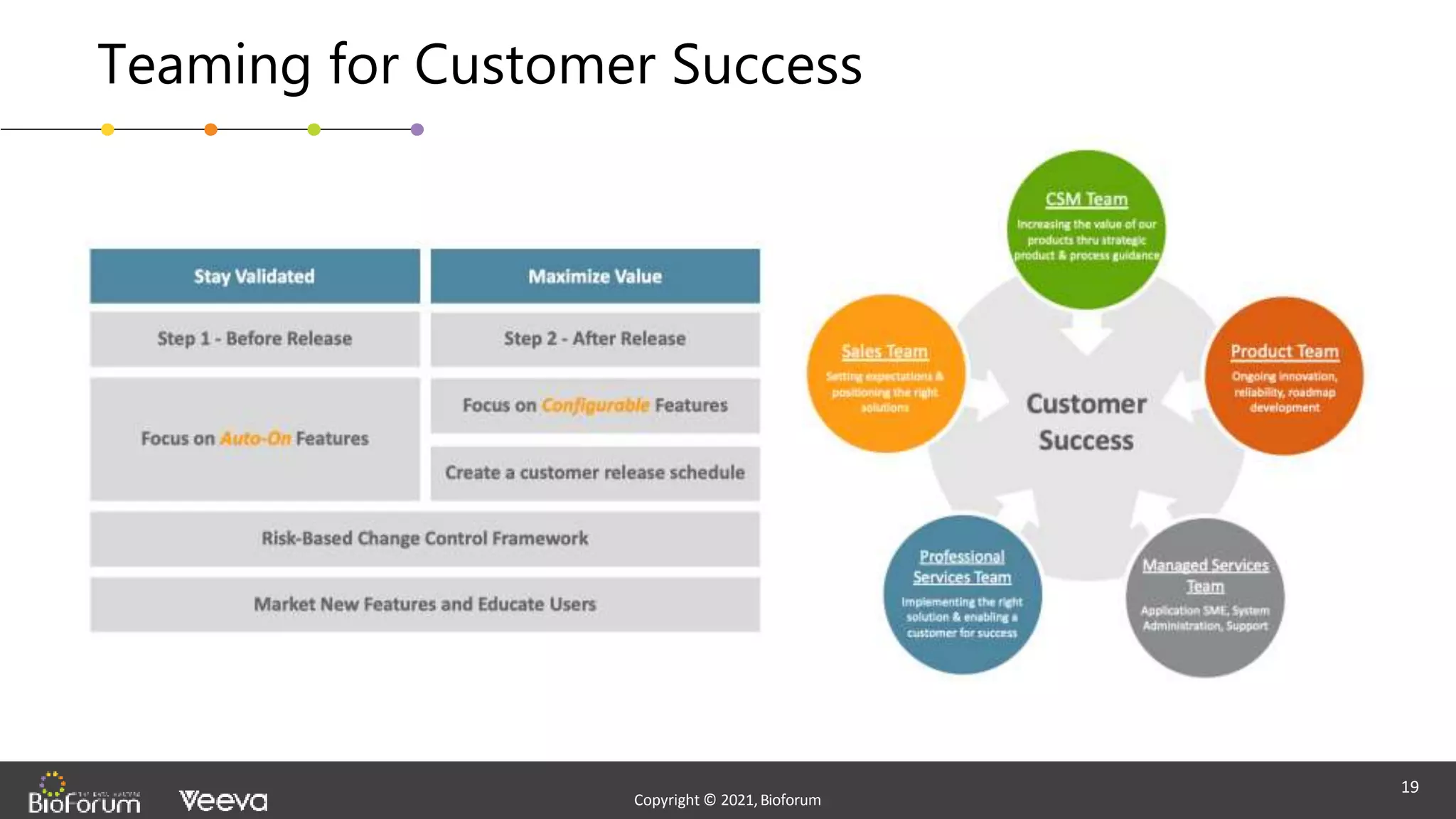
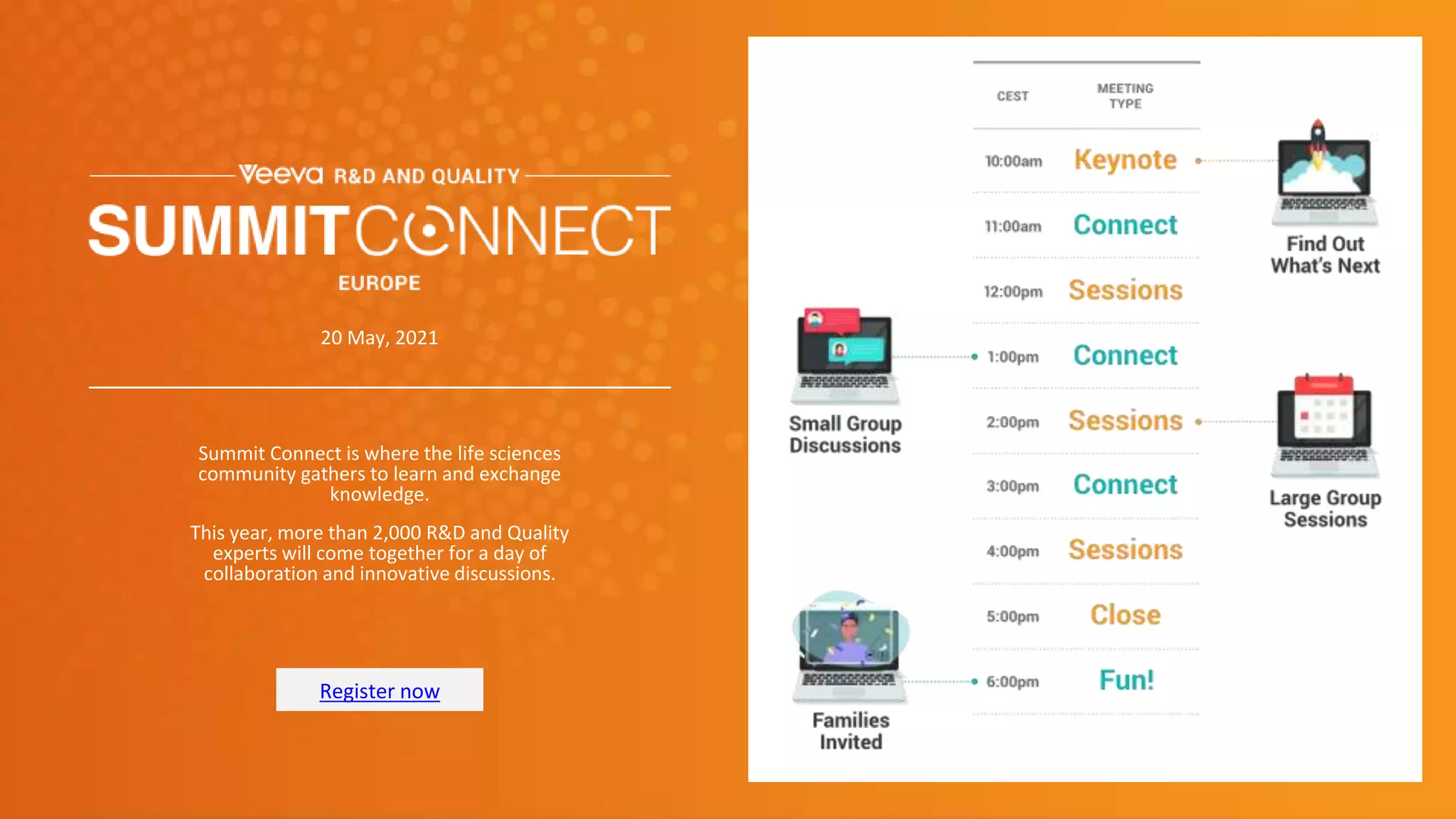
The document discusses validation strategies for cloud-based electronic data capture (EDC) systems, highlighting the importance of documenting validation processes to ensure data integrity and compliance with regulatory requirements. It addresses typical pitfalls in validation, outlines essential validation testing steps, and emphasizes the need for a structured approach to validate systems effectively. Additionally, it details the roles of the software vendor and the customer in the validation process to maintain a validated state throughout the system's lifecycle.